Abstract
Purpose
To evaluate the efficacy of intravitreal conbercept (IVC) in pars plana vitrectomy (PPV) for patients with proliferative diabetic retinopathy (PDR).
Methods
A meta-analysis of randomized control trials (RCTs) using online databases was performed. The intraoperative outcome measures were the incidence of intraoperative bleeding and endodiathermy application, and the mean surgical time. The postoperative outcome measures were mean change in best-corrected visual acuity (BCVA) from baseline, postoperative vitreous clear-up time and incidence of recurrent vitreous hemorrhage (VH).
Results
Eight RCTs were selected for meta-analysis. They included 409 eyes (215 eyes in IVC group and 194 eyes in no conbercept group). Preoperative IVC application was associated with less intraoperative bleeding and endodiathermy applications (RR = 0.34, 95% CI, 0.23–0.50, P < 0.00001, and RR = 0.26, 95% CI, 0.12–0.56, P = 0.0005) compared to no conbercept. It also shortened surgical time (WMD = −15.87, 95% CI, −22.04 to −9.69, P < 0.00001). In addition, preoperative or intraoperative IVC achieved better BCVA outcome (WMD = −0.37, 95% CI, −0.62 to −0.13, P = 0.003), shorter vitreous clear-up time postoperatively (WMD = −5.44, 95% CI, −6.31 to −4.57, P < 0.00001) and a lower rate of VH recurrence (RR = 0.45, 95% CI, 0.22–0.91, P = 0.03).
Conclusion
IVC is an effective adjuvant in PPV for PDR, with better intraoperative and postoperative outcomes.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a serious, vision-threatening ocular complication of diabetes mellitus, the leading cause of blindness in the working age population worldwide [1]. Proliferative diabetic retinopathy (PDR) is one of the leading causes of blindness in DR, characterized by neovascularization (NV), vitreous hemorrhage (VH), and tractional retinal detachment (TRD) [2]. Pars plana vitrectomy (PPV) is an established and successful treatment for severe complications of PDR, such as non-resolving VH and TRD [3]. Despite various technical improvements and instruments introduced over time, intraoperative bleeding usually happens during the epiretinal neovascular membrane peeling, which may require additional surgical maneuvers, increase the frequency of instrument exchange and prolong surgery time. Moreover, repeated bleeding could seriously affect visualization of the surgical field, thus greatly increase the occurring rate of complications like iatrogenic retinal break (IRB), which may lead to surgical failure [4].
In recent years, a variety of drugs have been used in PPV for PDR in order to reduce the probability of complications [4]. Although the exact mechanism has not yet been identified, anti-vascular endothelial growth factor (VEGF) agents have been used in PPV for PDR based on the observation that VEGF plays an important role in the development of retinal neovascularization and vitreous proliferation [5,6,7]. Bevacizumab (Avastin; Genentech Inc., San Francisco, CA), ranibizumab (Lucentis; Genentech Inc., San Francisco, CA), and aflibercept (Eylea; Regeneron Pharmaceuticals Inc., and Bayer Pharma AG, Berlin, Germany) are the most commonly used anti-VEGF agents as an adjunctive to vitrectomy for PDR, with excellent clinical outcomes [5, 6]. Another agent, conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China) was developed and approved by China Food and Drug Administration in 2013 for the treatment of age-related macular degeneration as in intravitreal injection. This relatively new drug is a VEGF receptor fusion protein, which can specifically bind to VEGF-A, VEGF-B, VEGF-C, and placental insulin-like growth factor (PlGF) [8]. The effect of conbercept on vitrectomy for PDR was first reported in 2016, showing that the application of intravitreal conbercept (IVC) before PPV could reduce the chances of intraoperative bleeding [7].
Previous meta-analyses of randomized controlled trials (RCTs) involving intravitreal injection of anti-VEGF agents before PPV for PDR have been focused mostly on the use of bevacizumab and ranibizumab, showing that the preoperative application of these anti-VEGF agents in patients with PDR could diminish intraoperative bleeding, shorten surgical time, and consequently improve the surgical outcomes [4, 9,10,11]. Only one study attempted a meta-analysis concerning the efficacy of IVC in PPV for PDR, showing that intravitreal conbercept was associated with a better postoperative best-corrected visual acuity (BCVA) improvement, better intraoperative outcome, and a lower rate of postoperative vitreous hemorrhage occurrence compared to no conbercept [12]. However, it included some retrospective studies and the comparative group included ranibizumab, which resulted in the inclusion of only three RCTs analyzing the use of conbercept vs. no conbercept. The availability of new reports prompted our decision to undertake an updated independent assessment of the available literature and to undertake a meta-analysis including only RCTs examining the efficacy of IVC in PPV for patients with PDR compared to PPV without conbercept (control group).
Materials and methods
Search strategy
We conducted a comprehensive search through PubMed, ISI Web of Science, and ClinicalTrials.gov, using the terms (“conbercept” or “KH902”) and (“diabetic retinopathy” or “proliferative diabetic retinopathy”), with the language restricted to English, up to April 2, 2020. Additional search was carried out by exploring reference lists in the originally identified articles.
Inclusion and exclusion criteria
The criteria we applied when published studies were considered eligible for this meta-analysis were: (1) study design: RCT, (2) population: patients undergoing vitrectomy for PDR, (3) intervention: preoperative or intraoperative IVC versus PPV without conbercept, and (4) outcome variables: (a) surgical time, (b) proportion of cases with intraoperative bleeding, endodiathermy application, and postoperative VH, (c) postoperative change in BCVA and the postoperative vitreous clear-up time. Articles reporting data from the same study, abstracts, letters to the editor, case reports, and review articles were excluded.
Outcome measures
The intraoperative outcome measures were the proportions of patients with intraoperative bleeding and endodiathermy application, and the mean surgical time. The postoperative outcome measures were the mean change in BCVA expressed as logarithm of the minimal angle of resolution (logMAR) from baseline, the postoperative vitreous clear-up time and the proportions of patients with recurrent VH.
Data extraction
Two experts (G.C. and W.L.) reviewed all entries generated by the search criteria and selected studies that matched the inclusion criteria, then extracted data from included studies. Conflicting assessments were discussed until consensus was reached. The list of extracted items was as follows: first author’s name, year of publication, country of origin, number of participants in each group, length of follow-up and outcome variables.
Qualitative assessment
Two reviewers (F.J. and S.M.) used the Jadad scale [13] to assess the methodological qualities of RCTs. This system is a 5-point scale with three items listed as follows: randomization, masking, and participant withdrawals/dropouts. Each item is assigned one point when randomization is mentioned and one additional point when the randomization method was judged to be appropriate. Similarly, one point is assigned when masking is mentioned and one additional point is added when the method of masking was deemed appropriate. Studies assigned fewer than three points were judged to be of poor methodologic quality.
Statistical analysis
The meta-analysis was conducted by using Cochrane Review Manager (RevMan) version 5.1 software. The weighted mean difference (WMD) was calculated for continuous data (e.g., mean surgical time). For dichotomous data (e.g., number of eyes), the risk ratio (RR) was calculated. P < 0.05 was considered statistically significant on the test for overall effect. Inter-study heterogeneity was estimated by the Q statistic-test [14]. If the Q statistic test turned out as statistically significant (P < 0.05), a random-effects model was used. In case where Q-statistic was not significant, a fixed-effects model was applied. Confidence intervals of the overall/pooled results are shown within square brackets in bold font on the left side of each forest plot. Publication bias was assessed by Begg’s rank correlation test and by Egger’s linear regression test with P < 0.05 considered significant [15, 16].
Results
Overall characteristics of selected trials and quality assessment
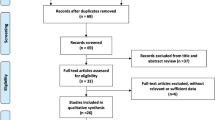
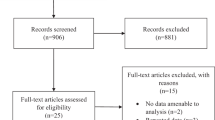
The search resulted in 251 entries being identified. Of these, 243 did not meet the inclusion criteria listed above and were rejected. This resulted in eight studies remaining which were included in this meta-analysis [7, 17,18,19,20,21,22,23]. Figure 1 depicts the overall study selection process. Outcome data were available from 409 eyes of 388 participants, comprising 215 eyes in the IVC group and 194 eyes in the control group, respectively. All included studies were RCTs and fulfilled our criterion of three or more points on the Jadad scale and all originated in China. Two studies administered conbercept immediately post vitrectomy [20, 21], and only the postoperative data were used for analysis. One study compared preoperative IVC injection alone (group 1) or combined with intraoperative IVC (group 3) to intraoperative IVC alone (group 2) [22]. Group 3 appeared to have higher proportion of patients with grade 3 vitreoretinal adhesion and a significantly lower portion with grade 1 vitreoretinal adhesion. Thus, only the intraoperative data between group 1 and group 2 were used for analysis. Other studies compared preoperative IVC injection to PPV without conbercept, and the intraoperative and postoperative data were used. Table 1 provides the characteristics and quality score of the included studies. Overall, all the average BCVA was balanced between the treatment group (IVC) and the control group in the six studies for which BCVA data were available; 1.83 (+/−0.42) for IVC group vs. 1.73 (+/−0.42) for the control group, equivalent to ~20/1350 vs. ~20/1070, respectively.
Intraoperative outcomes
Vitrectomy with preoperative use of IVC was associated with a lower rate of intraoperative bleeding (RR = 0.34, 95% CI, 0.23–0.50, P < 0.00001) and a lower number of endodiathermy applications (RR = 0.26, 95% CI, 0.12–0.56, P = 0.0005) compared to surgeries not using conbercept, with no heterogeneity identified (Fig. 2A, B). When overall surgical time was compared between groups, the IVC group also achieved a shorter overall surgical time of about 15.9 min (WMD = −15.87, 95% CI, −22.04 to −9.69, P < 0.00001). In this comparison, a heterogeneity was identified and, therefore, a random-effects model was applied to the data (Fig. 2C). It has to be pointed out that intraoperative IVC at the end of surgery in Gao et al. [22] may affect the surgical time duration, as most likely this procedure was counted as part of the overall surgical time, and a sub-analysis was conducted excluding this study to the rest of the studies for a comparison regarding mean surgical time. The sub-analysis confirmed the results from the main analysis, with the heterogeneity remaining significant (P = 0.01), while the mean difference in surgical time changes slightly (from ~15.9 min to ~17.2 min in favor of preoperative IVC), and the 95% CI remained below zero (−25.29 to −9.13) (Supplementary Appendix 1). Thus, the overall average reduction in surgical time remained at ~25% compared to the control group.
Only two studies reported additional outcomes (frequency of IRBs or application of silicone oil tamponade) [7, 18], the incidence of these complications was also in favor of the IVC group (RR = 0.21, 95% CI, 0.06–0.75, P = 0.02 and RR = 0.39, 95% CI, 0.23–0.67, P = 0.0005) (Fig. 3).
Postoperative outcomes
When preoperative or intraoperative IVC was compared to PPV alone in terms of mean change in logMAR BCVA from baseline, it showed superior results (WMD = −0.37, 95% CI, −0.62 to −0.13, P = 0.003)(Fig. 4A). Of note, the average preoperative acuity for the four studies analyzed was 1.98 logMAR for the IVC group vs. 1.82 logMAR for the control group, equivalent to ~20/1900 and ~20/1300, respectively, while the average postoperative BCVA was 0.64 logMAR for the IVC group vs. 0.87 logMAR for the control group (equivalent to ~20/90 vs. ~10/150, respectively).
Similarly, the vitreous clear-up time postoperatively was shorter for the IVC group vs. the control group (WMD = −5.44, 95% CI, −6.31 to −4.57, P < 0.00001), with no heterogeneity identified (Fig. 4B). In this meta-analysis, the mean vitreous clear-up time after vitrectomy in the IVC group was 5.9 days vs. 11.6 days in the control group, a mean reduction of ~50%.
The IVC group also achieved a lower rate of vitreous hemorrhage recurrence (RR = 0.45, 95% CI, 0.22–0.91, P = 0.03); however, heterogeneity was identified, and a random-effects model was applied to the data (Fig. 4C). Overall, the average incidence rate in the IVC group was ~25.4% vs. 57,1% in the control group, a 45% reduction.
Begg’s test and Egger’s test indicated no statistically significant evidence of publication bias for any of the parameters.
Discussion
In this meta-analysis, we reviewed eight RCTs, including 409 eyes (215 eyes in the IVC group and 194 eyes in the control group). In terms of the intraoperative outcomes, the results of our meta-analysis clearly demonstrated better outcomes of vitrectomy with IVC pretreatment, with less intraoperative bleeding and endodiathermy applications, and shorter overall surgical time. The incidence of IRBs and silicone oil tamponade was also in favor of the IVC group. In terms of the postoperative outcomes, preoperative or intraoperative IVC was also with better BCVA outcome, shorter vitreous clear-up time postoperatively, and less postoperative VH.
VEGF and placental growth factor (PlGF) are known to play important roles in the pathogenesis of PDR by inducing pre-retinal neovascularization and disrupting the blood–retinal barrier [24, 25]. Conbercept, a recombinant fusion protein binds to all VERG receptors (VEGF-A, VEGF-B, and VEGF-C) and also to PlGF [8]. A clinical study showed that the concentration of VEGF and PlGF is reduced in aqueous humor 7 days after IVC with a mean decrease of ~52% and ~38% for VEGF and PIGF, respectively) [19]. In the same study, the VEGF levels in the vitreous decreased by ~71%, while there was no statistically significant decrease in vitreous PIGF levels. In addition to decreasing VEGF levels, IVC may alter favorably the protein profile involved in the immune response in vitreous, platelet degranulation, complement activation, inflammation endocytosis, proteolysis, and heme scavenging in patients with PDR [26]. The safety of conbercept had been evaluated in patients with neovascular age-related degeneration, showing that all the patients could tolerate the injections with minimal side effects [27]. Thus, the application of IVC in patients with PDR before or immediately after PPV is likely beneficial as a way of increasing the effectiveness of the procedure.
The pooled results in our analysis demonstrated that vitrectomy with IVC preoperative application was associated with less intraoperative bleeding and endodiathermy and shorter surgical time, the results are similar compared to the effect of other anti-VEGF agents, such as bevacizumab and ranibizumab, and had been reported in many previous studies [9, 10]. Although a comparison with studies using other anti-VEGF agents would have provided a broader understanding of the relative effectiveness and potential advantages of conbercept, we decided to limit this meta-analysis to the use of conbercept only for two reasons. First, the use of the drug has been limited to date to Chinese patients, while the other drugs have achieved worldwide distribution. This would likely result in pharmacogenetic differences in the response [28], which would be difficult to predict and account for. Second, the current use of conbercept as an adjuvant to PPV in PDR is currently limited to an off-label use, which results in a lack of standardized recommendations for use, leading to variance in methodology, for example the time of drug administration, as demonstrated by the data presented in Table 1.
Retinal vessels are less likely to be damaged during delamination due to the regression of retinal neovascularization after IVC, which greatly reduces the likelihood of intraoperative bleeding [17]. Without endodiathermy and blood aspiration, the need for tool exchange would be reduced, which results in time saving during surgeries and this was confirmed by the meta-analysis as a significant (~25%) reduction in time. Another positive effect of IVS appeared as reduction in the rate of occurrence of intraoperative bleeding (−2.5 times). Less intraoperative bleeding may provide a clear retinal visualization, which in turn would result in a lower rate of IRB occurance [29]. The easier separation of epiretinal membrane from the retinal surface could also explain the lower rate of IRBs in the preoperative IVC group. Silicone endotamponade is always used in the cases of intraoperative complications like IRBs [7]. However, only two studies reported that IRBs and silicone endotamponade occurred more frequently in the vitrectomy alone group [7, 18], therefore, future larger sample size comparative clinical trials are needed to confirm this observation.
The adjuvant IVC resulted in better BCVA outcome compared to the control group without use of conbercept. Conbercept could reduce vascular permeability and decrease retinal thickness, which could be beneficial for the recovery of retinal function in the post-operative period [20]. Based on published cross-sectional studies, it was expected that IVC could shorten vitreous clear-up time after vitrectomy and lead to a decrease in the incidence of recurrent VH; both expectations were confirmed in the current meta-analysis. Conbercept could decrease angiogenesis, preventing the progression of neovascularization in the postoperative period, which may be attributed to the shortened vitreous clear-up time and less recurrent VH [20]. Although most of the conbercept injected preoperatively is likely removed during vitrectomy, the remaining amount in the eye may still have an impact. Only two studies reported results from studies using conbercept administration immediately after vitrectomy [20, 21]. Intraoperative IVC application could results in higher postoperative concentration, and the half-life of conbercept in the vitreous was estimated to be 4.2 days in rabbits [30], which may have a better effect on postoperative outcomes. However, from surgeon’s experience, giving conbercept after vitrectomy diminishes its intraoperative advantage and should be limited to patients with early-stage PDR [20]. For severe PDR, conbercept prior to vitrectomy should be considered, as intraoperative bleeding may be occurring less frequently after treatment with IVC. Only one study reported that the preoperative combined with intraoperative application of IVC resulted in a lower incidence of vitreous hemorrhage and retinal detachment compared to preoperative IVC alone or intraoperative IVC alone [22], although the differences were not statistically significant, and therefore, this observation would need additional clinical trials to be confirmed.
This work carries some limitations which should be acknowledged. One of the main limitations is that all included studies were carried out with small or very small sample sizes, which may affect the results. Second, a potential source of heterogeneity is different trial duration, with three studies having a follow-up duration of 3 months, while three studies had a follow-up of 6 months, and one study––12 months. Thus, RCTs of longer duration (at least 12 months) and larger sample size would be preferable to assess more definitively long-term efficacy of IVC for PDR. Another limitation is that all the studies included in this meta-analysis originated from a single country. Thus, additional studies when the use of the drug is expanded to other regions would be helpful to assess the efficacy and safety in other geographic regions. Furthermore, as mentioned above, the off-label use of the drug leads to a lack of standardization of the therapeutic regiment, leading to a variation in terms of time with respect to time of surgery, which may decrease the efficiency of IVC application in some cases.
In summary, the present meta-analysis confirmed the theoretical advantages of preoperative IVC over the PPV alone, in terms of less intraoperative bleeding and endodiathermy applications, and shorter overall surgical time. Preoperative or intraoperative IVC was also with better BCVA outcome, shorter vitreous clear-up time postoperatively, and less postoperative VH. Thus, IVC is an effective adjunct in PPV for PDR.
Summary
What was known before
-
Pars plana vitrectomy is an established and successful treatment for severe complications of proliferative diabetic retinopathy, such as non-resolving vitreous hemorrhage and tractional retinal detachment. But whether intravitreal conbercept could improve the surgery outcome for proliferative diabetic retinopathy is uncertain.
What this study adds
-
The application of intravitreal conbercept prior to vitrectomy in proliferative diabetic retinopathy patients reduced surgical time and facilitated the procedure. It also shortened vitreous clear-up time postoperatively and reduced the recurrence of vitreous haemorrhage, resulting in better vision acuity.
References
Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
Blankenship GW, Machemer R. Long-term diabetic vitrectomy results. Ophthalmology. 1985;92:503–6.
Zhao XY, Xia S, Chen YX. Antivascular endothelial growth factor agents pretreatment before vitrectomy for complicated proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Br J Ophthalmol. 2018;102:1077–85.
Pakzad-Vaezi K, Albiani DA, Kirker AW, et al. A randomized study comparing the efficacy of bevacizumab and ranibizumab as pre-treatment for pars plana vitrectomy in proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retin. 2014;45:52–524.
Raczyńska D, Lisowska KA, Pietruczuk K, et al. The level of cytokines in the vitreous body of severe proliferative diabetic retinopathy patients undergoing posterior vitrectomy. Curr Pharm Des. 2018;24:3276–81.
Su L, Ren X, Wei H, et al. Intravitreal conbercept (KH902) for surgical treatment of severe proliferative diabetic retinaopathy. Retina. 2016;36:938–43.
Lu X, Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 2015;9:2311–20.
Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina. 2015;35:1931–42.
Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156:106–15.
Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95:1216–22.
Pranata R, Vania A Intravitreal conbercept improves outcome in patients undergoing vitrectomy for proliferative diabetic retinopathy: a systematic review and meta-analysis. J Evid Based Med 2020; https://doi.org/10.1111/jebm.12379. [Epub ahead of print].
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Yang X, Xu J, Wang R, et al. A randomized controlled trial of conbercept pretreatment before vitrectomy in proliferative diabetic retinopathy. J Ophthalmol. 2016;2016:2473234.
Cui J, Chen H, Lu H, et al. Efficacy and safety of intravitreal conbercept, ranibizumab, and triamcinolone on 23-gauge vitrectomy for patients with proliferative diabetic retinopathy. J Ophthalmol. 2018;2018:4927259.
Zhou J, Liu Z, Chen M, et al. Concentrations of VEGF and PlGF decrease in eyes after intravitreal conbercept injection. Diabetes Ther. 2018;9:2393–8.
Ren X, Bu S, Zhang X, et al. Safety and efficacy of intravitreal conbercept injection after vitrectomy for the treatment of proliferative diabetic retinopathy. Eye (Lond). 2019;33:1177–83.
Jiang T, Gu J, Zhang P, Chen W, Chang Q. The effect of adjunctive intravitreal conbercept at the end of diabetic vitrectomy for the prevention of post-vitrectomy hemorrhage in patients with severe proliferative diabetic retinopathy: a prospective, randomized pilot study. BMC Ophthalmol. 2020;20:43.
Gao S, Lin Z, Chen Y, et al. Intravitreal conbercept injection as an adjuvant in vitrectomy with silicone oil infusion for severe proliferative diabetic retinopathy. J Ocul Pharm Ther. 2020;36:304–10.
Li B, Li MD, Ye JJ, Chen Z, Guo ZJ, Di Y. Vascular endothelial growth factor concentration in vitreous humor of patients with severe proliferative diabetic retinopathy after intravitreal injection of conbercept as an adjunctive therapy for vitrectomy. Chin Med J (Engl). 2020;20:664–9.
Wisniewska-Kruk J, Klaassen I, Vogels IM, et al. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014;122:123–31.
Morera Y, González R, Lamdan H, et al. Vaccination with a mutated variant of human vascular endothelial growth factor (VEGF) blocks VEGF-induced retinal neovascularization in a rabbit experimental model. Exp Eye Res. 2014;122:102–9.
Zou C, Zhao M, Yu J, et al. Difference in the vitreal protein profiles of patients with proliferative diabetic retinopathy with and without intravitreal conbercept injection. J Ophthalmol. 2018;2018:7397610.
Li X, Xu G, Wang Y, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–7.
Chen G, Tzekov R, Li W, Jiang F, et al. Pharmacogenetics of complement factor H Y402H polymorphism and treatment of neovascular AMD with anti-VEGF agents: a meta-analysis. Sci Rep. 2015;5:14517.
El-Sabagh HA, Abdelghaffar W, Labib AM, et al. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology. 2011;118:636–41.
Li H, Lei N, Zhang M, Li Y, Xiao H, Hao X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp Eye Res. 2012;97:154–9.
Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205.
Funding
Supported by the Science Research Foundation of Aier Eye Hospital Group (No.AM1901D3 to WS Li.); Innovation Guidance Project of Science and Technology Department of Hunan Province (No.2018SK50102 to WS Li) and Welfare Technology Applied Research Program Fund of Science Technology Department of Zhejiang Province (No.LGF18H120002 to YH Tong.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, G.H., Tzekov, R., Mao, S.H. et al. Intravitreal conbercept as an adjuvant in vitrectomy for proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Eye 36, 619–626 (2022). https://doi.org/10.1038/s41433-021-01474-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01474-5
This article is cited by
-
Influencing factors of low vision 2 years after vitrectomy for proliferative diabetic retinopathy: an observational study
BMC Ophthalmology (2023)
-
Downregulation of angiogenic factors in aqueous humor associated with less intraoperative bleeding in PDR patients with NVG receiving conbercept: a randomized controlled trial
BMC Ophthalmology (2022)