Abstract
Cancer patients are susceptible to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Different antitumor treatments have attracted wide attention in the context of coronavirus disease 2019 (COVID-19), especially immune checkpoint inhibitors (ICIs) that have revolutionized oncology changes. It may also have protective and therapeutic roles in viral infections. In this article, we collected 26 cases of SARS-CoV-2 infection during ICIs therapy and 13 related to COVID-19 vaccination from Pubmed, EMBASE, and Wed of Science. Of these 26 cases, 19 (73.1%) presented mild cases and 7 (26.9%) were severe cases. Melanoma (47.4%) was a common cancer type in mild cases and lung cancer (71.4%) in severe cases (P = 0.016). The results showed that their clinical outcomes varied widely. Although there are similarities between the immune checkpoint pathway and COVID-19 immunogenicity, ICIs therapy overactivated T cells, which often leads to immune-related adverse events. In fact, the COVID-19 vaccine has been shown to be safe and effective in patients treated with ICIs. In this review, we report the vital clinical observations of SARS-CoV-2 infection or vaccination in cancer patients treated with ICIs and explore the potential interaction between them.
Similar content being viewed by others
Introduction
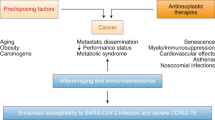
The coronavirus disease 2019 (COVID-19) pandemic has affected almost every country, community, and a large number of individuals. Cancer patients, due to the tumor itself and various anticancer treatments, are immunocompromised and thus more susceptible to being infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1, 2]. Patients with cancer had higher rates of intensive care unit (ICU) admissions and higher mortality compared with COVID-19 patients without cancer [2]. The impact of specific cancer therapies varied also aroused widespread concern during the pandemic, particularly in immune checkpoint inhibitors (ICIs).
ICIs, including anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), anti-programmed cell death protein-1 (PD-1), and anti-PD-ligand 1 (PD-L1), have shown higher efficacy against some solid tumors by clearing the inhibitory pathways that block effective anti-tumor T-cell responses [3,4,5] and are a recent promising cancer treatment. The impact of ICIs is lasting. Gatto et al. hypothesized that cancer patients undergoing ICIs could enhance their ability against SARS-CoV-2 by increasing the activity of T cells [6], but ICIs also disrupt immune homeostasis and may lead to immune-related adverse events (irAEs) [7], which may increase the difficulties of diagnosis in the context of COVID-19.
The popularity of vaccines has brought a new dawn to cancer patients. Studies based on influenza vaccines have shown that influenza vaccines are safe in ICIs-treated patients, and no new or higher levels of irAEs have been observed after vaccination [8, 9]. In fact, irAEs are known to occur in cancer patients who receive ICIs. Due to the overstimulation of the immune system during vaccination, it remains imperative to consider the possibility of irAEs and exercise caution when treating such patients [10].
Clinical performance, treatment and prognosis of cancer patients treated with ICIs after SARS-CoV-2 infection or COVID-19 vaccination are divergent. The immune response between them is still the focus of research. This literature review aimed to summarize the published cases, elaborate on their clinical features and prognosis, and discuss the potential interaction between them.
COVID-19 immunology
COVID-19 is an immune-related disease. The invasion of SARS-CoV-2 rapidly activated various immune cells and facilitated the generation of the protective immune response, including the activation and expansion of CD4+ and CD8+T cells. Generally eliminating virus infection and gaining effective immunity depends on natural killer cells and cytotoxic CD8+T [11], the latter through eliminates infected cells by releasing cytotoxic granules such as granzymes, perforin [12]. Several studies have shown that sustained viral stimulation may induce T cells in COVID-19 patients to become dysregulated, hyperactivated, and subsequently exhausted [13, 14]. Diao et al. found that the number of CD4+T and CD8+T cells decreased dramatically in COVID-19 patients, especially for patients admitted to ICU [15]. Decreased CD8+T and natural killer cells were also found but highly activated in severe COVID-19 [16], which partly explains the severe immune damage [17]. T cell exhaustion may be one of the major causes of worsened clinical outcomes in COVID-19 patients [12].
Immune checkpoint (IC) molecule PD-1 plays a central role in peripheral activated T cells, and its expression is considered to be a marker of T cell exhaustion [18]. Levels of PD-1 and CTLA-4 expressed on both CD4+T and CD8+T were observed to be upregulated in COVID-19 patients [19, 20], which may reflect an association with the severity of COVID-19 [20]. Shahbaz et al. indicated that PD-1 expression was much higher in severe COVID-19 than in mild/moderate disease [19]. Similarly, Avendano-Ortiz et al. proved that IC can be used as a marker to determine the severity of COVID-19 at admission [21].
During SARS-CoV-2 infection, immune effector cells were activated and a large number of cytokines and chemokines are synthesized and released. It has been observed that the level of interleukin (IL)-1, IL-6, interferon (IFN)-γ, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor (TNF) were significantly increased in patients with severe COVID-19 [22]. High concentrations of IL-6, IL-10, and IFN-γ were confirmed to negatively regulate T cell survival or proliferation and play a key role in inducing lymphopenia [15]. To compensate for the disadvantage of depleted lymphocytes, more proinflammatory cytokines were secreted by activated macrophages, neutrophils, and monocytes [23], further promoting the production of cytokine storm, which accelerates the progression of patients to acute respiratory distress syndrome (ARDS) and multiple organ dysfunction. High IL-6 levels exert pleiotropic effects on the immune system through cis-signaling, resulting in cytokine release syndrome (CRS) [24]. IL-6 inhibitor has been shown to be effective in patients with severe COVID-19 and have become one of the standard treatments against COVID-19, but the best beneficiary group needs to be evaluated [25].
ICIs mechanism of action
In cancer and chronic infection condition, due to prolonged exposure to the same antigen, T cells gradually lose their effect and show overexpression of IC molecules [26]. ICIs reverse T cell exhaustion by blocking immunosuppressive signaling between antigen-presenting cells and T cells, thus enhancing effective immune protection [27]. Specifically, CTLA-4 competes with CD28 for ligand B7 expressed on antigen-presenting cells to control previously activated T cells. Scientists observed that anti-CTLA-4 not only enhances the function of T cells but also reduces the regulatory T cells [3]. PD-1 binding to PD-L1 is responsible for blocking the proliferation and survival of cytotoxic CD8+T. The purpose of ICIs is not to kill tumor cells directly, but to enhance immune response and endogenous antitumor activity by anti-CTLA-4 to increase co-stimulation or blocking PD-1/PD-L1 to inhibit the induced death of effector T cells [18]. The difference in the mechanism of action between CTLA-4 and PD-1 shows the potential for combination therapy. A randomized, double-blind, phase 2 trial revealed that 4-year recurrence-free survival was significantly higher in the nivolumab plus ipilimumab group (64.2%) than the nivolumab alone group (31.4%) or placebo group (15.0%) [28]. But in some cases, this combination therapy did not improve disease-free survival [29]. Thus, clarifying the indications and determining the mechanism of administration is necessary for combination therapy.
Notably, activation of the immune system by ICIs may impair tolerance to certain normal tissue antigens and cause irAEs, thereby affecting multiple organs including skin, gastrointestinal tract, liver, and pancreas [30]. In very rare cases, the effects can be fatal. Researchers found that PD-1 combined with CTLA-4 blockade triggers more irAEs than monotherapy, and the most common cause of death is colitis [31]. irAEs usually occur within the first few weeks to months of treatment initiation, but delayed toxicity can occur even after treatment is stopped [30]. The exact mechanisms of irAEs are uncertain, but it is thought to be related to specific T cell response, B cell activation, autoantibodies and cytokine-mediated breakdown of self-tolerance [32, 33]. In addition, researchers noticed that treatment of irAEs such as corticosteroids, target TNF-α drugs may increase the risk of opportunistic infections [26]. Therefore, it is necessary to carry out medical suspicion when the clinical condition deteriorates after receiving these additional immunosuppressive therapies to correct irAEs [26].
Clinical characteristics of SARS-CoV-2 infection in cancer patients treated with ICIs
We performed the term (‘SARS-CoV-2’ OR ‘COVID-19’ OR ‘2019-ncov’ OR ‘novel coronavirus’ OR ‘coronavirus’) and (‘immune checkpoint inhibitor’ OR ‘immunotherapy’ OR ‘ipilimumab’ OR ‘nivolumab’ OR ‘pembrolizumab’ OR ‘cemiplimab’ OR ‘avelumab’ OR ‘durvalumab’ OR ‘atezolizumab’) to search in PubMed, EMBASE, and Web of Science for articles published in English between March 2020 to March 2023.
26 cases of SARS-CoV-2 infection during ICIs therapy were collected from 19 research centers (Table 1), based on the degree of disease progression, ICU admission or intubation, we divided 26 patients into mild (73.1%) [34,35,36,37,38,39,40,41,42,43,44,45] and severe cases (26.9%) [46,47,48,49,50,51,52]. Of them, 65.4% were male, and the median age was 62.0 (22–83) years. According to the medical history, 38.5% had melanoma, 30.8% had lung cancer, 23.1% had tumors of the urinary system, 3.85% had hematologic malignancies and 3.85% had Merkel cell cancer. And we found melanoma (47.4%) was common in mild cases, while more lung cancer (71.4%) patients were found in severe cases (P = 0.016) (Table 2). 65.4% received anti-PD-1 monotherapy, 23.1% received anti-CTLA-1/PD-1 (nivolumab/ipilimumab) combination therapy, and 11.5% received anti-PD-L1 (atezolizumab) therapy. 65.4% presented with fever as an initial presentation, followed by cough (50.0%) and dyspnea (38.5%). The time from the last treatment cycle of ICIs to the diagnosis of SARS-CoV-2 infection ranged from 2 to 56 days (median: 18). Laboratory tests reported elevated C-reactive protein in 17 (65.4%) patients and decreased lymphocyte counts in 9 (34.6%).
In terms of treatment, 53.8% of patients received antibiotics, 34.6% with hydroxychloroquine, and 19.2% with antiviral therapy. 4 patients were due to mild conditions and recovered through self-isolation instead of specific treatment. The prognosis of severe patients was significantly worse than that of mild patients (P = 0.007). Ultimately, five severe cases died, and two mild cases died of severe neurological deterioration and chronic heart disease, respectively. Of the remaining patients, ten were reported to have resumed or planned to restart immunotherapy.
Clinical characteristics of COVID-19 vaccination in cancer patients treated with ICIs
Similarly, we searched for cases in which patients who had previously used ICI received COVID-19 vaccine. A total of 13 cases of adverse reactions following the COVID-19 vaccination were reported in cancer patients treated with ICIs (Table 3) [53,54,55,56,57,58,59,60,61,62,63,64,65]. The median age was 55.5 (25–75) years, and males accounted for 53.8%. Of them, the most common cancer subtype was lung cancer (46.1%), followed by melanoma (30.8%), hepatocellular cancer (7.7%), colorectal cancer (7.7%) and parotid cancer (7.7%). 61.5% had previously received anti-PD-1 monotherapy, 30.8% received anti-CTLA-1/PD-1 (nivolumab/ipilimumab) combination therapy, and 7.7% received anti-PD-L1 (durvalumab) therapy. The median time from the last ICI dose to the onset of disease after vaccination was 11 (3–90) days. 4 patients (30.8%) had irAEs before the COVID-19 vaccination. All patients were accepted with mRNA vaccines, 6 (46.2%) patients develop symptoms after the first dose of vaccine, 5 (38.5%) patients developed clinical symptoms after the second dose, and 2 (15.4%) cases after the third dose. The median time from vaccination to onset was 3 (2–14) days. The clinical presentation of the patients varied, of which three were diagnosed with type 1 diabetes mellitus (T1DM), three with cutaneous complications, two with CRS, and one each with necrotizing myopathy, thrombocytopenic purpura, hepatitis, encephalitis and tumor relapse. Of 13 patients, 12 were improved and discharged after treatment with steroids or insulin, and symptomatic treatment.
Interaction between ICIs and SARS-CoV-2 infection
Up to now, the data on ICI-treated cancer patients with co-infection with SARS-CoV-2 is limited. The restoration of cellular immunity by anti-PD-1 has been shown to reactivate the depleted antiviral T-cell response and lower viral load [66]. Among the 652 melanoma patients treated with ICI registered in the German working group of dermato-oncology, only 13 were found to be COVID-19 positive, and most of them had mild symptoms [67]. As our case series summarized, COVID-19 patients treated with ICIs do not necessarily have a serious course of disease [44]. In case 14 and 19, although two patients were positive for SARS-CoV-2 PCR testing, neither of them showed obvious signs of lung involvement. It is speculated that the cause for this mild condition may be related to the blockage of the PD-1/PD-L1 pathway [43, 45].
A previous study pointed out that patients who received ICI during influenza infection might be more immunocompetent than those receiving chemotherapy patients [68]. ICI targets IC receptors on T cells, increases CD8+T cell activity, and activates immune cells, thus enhancing antiviral immune response, accelerating virus clearance, and ultimately phagocytosis and destroying virus-infected cells [69] (Fig. 1). Gatto et al. suggested that cancer patients undergoing ICI are more ‘resistant’ to SARS-CoV-2 attacks [6]. Similarly, Yatim et al. demonstrated that melanoma patients treated by ICI showed increased T cell activation during SARS-CoV-2 infection [70], leading us to believe that cancer patients receiving ICI may recover more successfully in COVID-19 cases. Of note, T cell exhaustion in severe COVID-19 is irreversible, ICI may only play a role in the low or medium level of PD-1 [71]. Therefore, the recovery of immunity in cancer patients receiving ICI may not be sufficient to protect these patients from severe COVID-19.
The clinical status of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients receiving ICI remains uncertain, but they may have potential interactions. ICI blocks the inhibitory pathway to enhance immunity, increase the release of cytokines, accelerate virus clearance, and ultimately phagocytosis and destroy virus-infected cells. On the other hand, the overactivated immune response may interact with T depletion and cytokine storm in severe COVID-19 patients to induce cytokine release syndrome and accelerate the progression of ARDS [69]. The increased expression of immune checkpoint receptors in turn increases the number of regulatory T cells, which promotes tumor progress [71]. ARDS acute respiratory distress syndrome, COVID-19 coronavirus disease 2019, CTLA-4 cytotoxic T lymphocyte-associated antigen 4, CRS cytokine release syndrome, IFN-γ interferon-γ, IL interleukin, PD-1 programmed cell death protein-1, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, Treg regulatory T cells, TNF tumor necrosis factor.
Early hypotheses postulated that overactivation of immunity in patients receiving immunotherapy leads to CRS, which possibly aggravates COVID-19 disease [6, 7]. The plausible explanation for this view is related to the mechanism of further damage of respiratory epithelium caused by overactivation of T cells [17]. Murata et al. reported a 70-year-old man who had been treated with nivolumab and ipilimumab for lung cancer and was diagnosed with CRS due to systemic symptoms with inflammation and elevated IL-6 after SARS-CoV-2 infection [51]. The authors deem that the patient showed a good response to immunotherapy, and the occurrence time of CRS coincided with the time of SARS-CoV-2 infection. It is considered that CRS may be irAEs caused by infection [51].
CRS is a systemic inflammatory disease that begins with fever and is featured by high cytokine release, especially for IL-6, triggered by infection or medication [72]. Murata et al. summarized previously reported CRS cases induced by ICIs, and IL-6 levels varied greatly among different cases [51]. The research found that IFN-γ enhances IL-6 production in monocytes [73] and involved in the development of ARDS in COVID-19 [22], while IFN signaling is the pathway leading to PD-1/PD-L1 expression [74]. Nevertheless, there is no significant correlation between previous use of ICI and the severity of COVID-19, as evidenced by current research [70, 75]. The probability of CRS after ICIs use has been reported to be approximately 0.06%-0.14% [76]. Cytokine storms are more common in severe COVID-19 patients, and the worst-case condition may cause ARDS, but the probability is extremely small (Fig. 1).
In addition, ICI immune-mediated lung injury and COVID-19 have overlapping features and common clinical and radiological manifestations, making it not only difficult to distinguish between the two but also impossible to exclude a negative synergistic effect of both on lung injury [77]. Taken together, clinicians need to be more cautious with ICI-treated cancer patients during COVID-19.
Interaction between ICIs and COVID-19 vaccination
COVID-19 vaccine is recognized by various innate sensors after injection, leading to cell activation and production of type I INF, which further promotes T cell activation and differentiation into effector cells that exert their effects, as evidenced by significant antibody titers and specific antibody responses [78]. However, vaccine induce immune responses may be affected by different cancer treatments, in which patients receiving immunotherapy or targeted therapy are more likely to develop seropositive status than patients receiving cytotoxic chemotherapy [79]. The results of the systematic evaluation by Ruiz et al. showed that COVID-19 vaccines are effective in ICI-exposed patients and they had higher seroconversion rates than those receiving chemotherapy [80]. In another prospective study of immune responses to mRNA-1273 COVID-19 vaccination in patients with different anticancer therapies, antibody concentrations were lower in all cancer patients than in non-cancer controls on day 28 after the second vaccination [81]. But notably, 122 of the 131 patients treated with ICI showed an adequate response, which was the highest probability of all cancer cohorts [81].
On the other hand, in view of the occurrence of irAEs after ICI therapy, the safety of COVID-19 vaccination should be considered. Kian et al. pointed out that none of the different cancer treatments showed any effect on the development of adverse events after COVID-19 vaccination, while patients receiving ICI also showed no more side effects than other treatments [82]. A survey of 134 patients treated with ICI in two medical centers in Israel also found no new immune-related side effects. Importantly, vaccine-related side effects were mild even in patients with previous immune-related adverse reactions [83]. Mei et al. observed that vaccinated individuals may have milder irAEs compared to unvaccinated people (P < 0.001) in ICI treated patients, but no difference was observed in serious irAEs [84]. Furthermore, the authors suggested the optimal window between anti-PD-1 therapy and COVID-19 vaccination might be >16 days [84]. Another observational study found that skin cancer patients treated with ICIs were tolerant to the COVID-19 vaccine. The authors compared the occurrence of irAEs before and after vaccination and found that 17 and 15 patients developed irAEs, respectively, but all patients responded well to corticosteroids [85]. Intriguingly, authors also observed patients with shorter intervals between vaccination and ICI were more likely to develop side effects [85], suggesting determining whether the time span between the two is related to the occurrence of adverse reactions may be an area for future research. The accumulated evidence suggests that it is easy to see that COVID-19 vaccination is effective and safe in cancer patients treated with ICIs.
Nevertheless, in theory, both immunotherapy and COVID-19 vaccines can trigger inflammatory and immune responses. Several COVID-19 vaccines induced autoimmune diseases had been reported, such as immune thrombotic thrombocytopenia, autoimmune liver diseases and T1DM [86]. In our case series, 13 patients treated with ICI had adverse reactions after COVID-19 vaccination, which are very rare but still exist.
Several cases describe a unique temporal relationship between the occurrence of irAEs and COVID-19 vaccination [53, 56, 61, 63]. Scientists considered the COVID-19 vaccine, as a potential trigger, may promote the development of irAEs in the context of immunotherapy [54, 57, 58] (Fig. 2). Au et al. reported a colorectal patient who received long-term PD-1 inhibitor therapy and was diagnosed with CRS after the first dose of COVID-19 [53]. Due to the sequence similarity between spike proteins and new tumor antigens, T cells resident in tissues or lymph nodes cause CRS through cross-reaction, although it is less likely [53]. It has been demonstrated that CRS rarely occurs after COVID-19 vaccination under cancer immunotherapy [87].
ICI induces T cells proliferation and enhances effect function, COVID-19 vaccination increases co-stimulation between antigen presenting cells and T cells receptors, which as a potential stimulator, which may induce the occurrence of irAEs. Increased cytokine release also involved immune events. CTLA-4 cytotoxic T lymphocyte-associated antigen 4, CRS cytokine release syndrome, IFN interferon, IL interleukin, irAEs immune-related adverse events, MHC major histocompatibility complex, PD-1 programmed cell death protein-1, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, T1DM type 1 diabetes.
In addition, inhibition of the PD-1/PD-L1 pathway leads to excessive proliferation of T cells and autoimmune activation, most nivolumab-related T1DM complications occur within 7 months after the first injection [88]. We identified three published cases of ICI-treated cancer patients who developed symptoms within days of their second COVID-19 vaccination and were subsequently diagnosed with T1DM [59, 61, 62]. This temporal difference may have other triggers. The incidence of T1DM is not high in COVID-19 patients under 30 years old [89], but the cause of new-onset hyperglycemia may be related to direct viral invasion of pancreas β cells and proinflammatory cytokine response caused by SARS-CoV-2 infection [90], it is reasonable to assume that similar reactions may occur after SARS-CoV-2 antigen presentation following vaccination [91]. COVID-19 vaccines induced T cell and B cell expansion and increased cytokine secretion. mRNA vaccines appear to have adjuvant properties and induce an immune response that may induce stronger CD4+, CD8+T cell reactions compared with traditional vaccines [92]. T cells were known to be involved in T1DM. Thus, it cannot rule out the synergistic effect of vaccination and ICI-induced irAEs (Fig. 2). Long-term clinical and immunological analyses are needed to understand the potential interaction between ICIs therapy and COVID-19 vaccination.
Of course, this paper has some limitations. The potential mechanisms described above have not been confirmed and are only a hypothesis based on the combination of cases. Secondly, there is little literature on COVID-19 vaccination of cancer patients receiving immunotherapy, and there may be many unreported cases, which does not represent the true incidence of irAEs and the causal relationship between disease and vaccine and ICI cannot be determined from these single cases.
Conclusion
Cancer patients are thought at high risk for COVID-19. It seems prudent to comprehensively assess risk factors and symptoms of SARS-CoV-2 infection in all patients who have received or are receiving ICI therapy and to screen for SARS-CoV-2 PCR early for a definitive diagnosis, especially for lung cancer patients. The benefits of vaccination against SARS-CoV-2 in these patients outweigh the risk, and still encouraged to be vaccinated. Clinicians should pay attention to the time window between ICIs and vaccination, as well as the follow-up after vaccination. Larger studies with longer follow-ups are still needed to fully assess the benefits and harms of the SARS-CoV-2 infection and COVID-19 vaccine in ICI-treated patients.
References
Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18.
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–91.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl J Med. 2017;377:1345–56.
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28.
Gatto L, Franceschi E, Nunno VD, Brandes AA. Potential protective and therapeutic role of immune checkpoint inhibitors against viral infections and COVID-19. Immunotherapy. 2020;12:1111–4.
Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269–73.
Gwynn ME, DeRemer DL, Saunders KM, Parikh J, Bollag RJ, Clemmons AB. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J Oncol Pharm Pr. 2020;26:647–54.
Valachis A, Rosen C, Koliadi A, Digkas E, Gustavsson A, Nearchou A, et al. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology 2021;10:1886725.
Retnakumar SV, Chauvin C, Bayry J. The implication of anti-PD-1 therapy in cancer patients for the vaccination against viral and other infectious diseases. Pharm Ther. 2023;245:108399.
Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490–500.
Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021;162:30–43.
Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–3.
De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11:3434.
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.
Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, et al. COVID-19 pneumonia: CD8+ T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218:108516.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61.
Shahbaz S, Xu L, Sligl W, Osman M, Bozorgmehr N, Mashhouri S, et al. The quality of SARS-CoV-2-specific T cell functions differs in patients with mild/moderate versus severe disease, and T cells expressing coinhibitory receptors are highly activated. J Immunol. 2021;207:1099–111.
Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, Al Heialy S, Alsafar HS, Hamoudi R, et al. Enhanced expression of immune checkpoint receptors during SARS-CoV-2 viral infection. Mol Ther Methods Clin Dev. 2021;20:109–21.
Avendano-Ortiz J, Lozano-Rodriguez R, Martin-Quiros A, Terron V, Maroun-Eid C, Montalban-Hernandez K, et al. The immune checkpoints storm in COVID-19: role as severity markers at emergency department admission. Clin Transl Med. 2021;11:e573.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Fathi N, Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol Int. 2020;44:1792–7.
Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473–4.
Plocque A, Mitri C, Lefevre C, Tabary O, Touqui L, Philippart F. Should we interfere with the interleukin-6 receptor during COVID-19: what do we know so far? Drugs 2023;83:1–36.
Lasagna A, Cassaniti I, Sacchi P, Baldanti F, Bruno R, Pedrazzoli P. Infectious complications and immunotherapy: old pitfalls and new horizons. Future Oncol. 2022;18:2377–81.
Lesch S, Gill S. The promise and perils of immunotherapy. Blood Adv. 2021;5:3709–25.
Livingstone E, Zimmer L, Hassel JC, Fluck M, Eigentler TK, Loquai C, et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): final results of a randomised, double-blind, phase 2 trial. Lancet 2022;400:1117–29.
Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet 2023;401:821–32.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N. Engl J Med. 2018;378:158–68.
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8.
Konig D, Laubli H. Mechanisms of immune-related complications in cancer patients treated with immune checkpoint inhibitors. Pharmacology 2021;106:123–36.
Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. 2022;71:169–78.
Anastasopoulou A, Gkoufa A, Diamantopoulos P, Kazanas S, Eliadi I, Samarkos M, et al. Clinical course of COVID-19 infection in a melanoma patient treated with nivolumab and bempegaldesleukin: a case report. Immunotherapy 2022;14:1015–20.
Arenbergerova M, Gkalpakiotis S, Arenberger P, Fialova A, Pasek M. COVID-19 in 3 patients treated with immune checkpoint inhibitors for advanced melanoma. J Dermatol Treat. 2022;33:1782–3.
Artigas C, Lemort M, Mestrez F, Gil T, Flamen P. COVID-19 pneumonia mimicking immunotherapy-induced Pneumonitis on 18F-FDG PET/CT in a patient under treatment with Nivolumab. Clin Nucl Med. 2020;45:e381–e2.
de Joode K, Oostvogels AAM, GeurtsvanKessel CH, de Vries RD, Mathijssen RHJ, Debets R, et al. Case report: adequate T and B cell responses in a SARS-CoV-2 infected patient after immune checkpoint inhibition. Front Immunol. 2021;12:627186.
Di Giacomo AM, Gambale E, Monterisi S, Valente M, Maio M. SARS-COV-2 infection in patients with cancer undergoing checkpoint blockade: clinical course and outcome. Eur J Cancer. 2020;133:1–3.
Bonomi M, Maltese M, Brighenti M, Muri M, Passalacqua R. Tocilizumab for COVID-19 pneumonia in a patient with non-small-cell lung cancer treated with chemoimmunotherapy. Clin Lung Cancer. 2021;22:e67–e9.
O'Kelly B, McGettrick P, Angelov D, Fay M, McGinty T, Cotter AG, et al. Outcome of a patient with refractory Hodgkin lymphoma on pembrolizumab, infected with SARS-CoV-2. Br J Haematol. 2020;190:e1–e3.
Pala L, Conforti F, Cocorocchio E, Ferrucci P, De Pas MT, Stucchi S, et al. Course of Sars-CoV2 infection in patients with cancer treated with anti-PD-1: a case presentation and review of the literature. Cancer Invest. 2021;39:9–14.
Rolfo C, Cardona AF, Ruiz-Patino A, Ariza S, Zatarain-Barron L, Pino LE, et al. Atypical skin manifestations during immune checkpoint blockage in coronavirus disease 2019-infected patients with lung cancer. J Thorac Oncol 2020;15:1767–72.
Schmidle P, Biedermann T, Posch C. COVID-19 in a melanoma patient under treatment with checkpoint inhibition. J Eur Acad Dermatol Venereol. 2020;34:e465–e6.
Szabados B, Abu-Ghanem Y, Grant M, Choy J, Bex A, Powles T. Clinical characteristics and outcome for four SARS-CoV-2-infected cancer patients treated with immune checkpoint inhibitors. Eur Urol. 2020;78:276–80.
Yekeduz E, Dursun B, Aydin GC, Yazgan SC, Ozturk HH, Azap A, et al. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J Oncol Pharm Pr. 2020;26:1289–94.
Ahmed MS, Brehme H, Friedrich S, Reinhardt L, Blum S, Beissert S, et al. COVID-19 and immune checkpoint inhibitors. J Eur Acad Dermatol Venereol. 2021;35:e312–e4.
da Costa CM, de Souza ZS, Real Salgues AC, Harada G, Marino Rodrigues Ayres PP, Vieira Nunes DB, et al. COVID-19 in a patient with advanced Merkel cell carcinoma receiving immunotherapy. Immunotherapy 2020;12:1133–8.
Di Noia V, D'Aveni A, Squadroni M, Beretta GD, Ceresoli GL. Immune checkpoint inhibitors in SARS-CoV-2 infected cancer patients: the spark that ignites the fire? Lung Cancer. 2020;145:208–10.
Bonomi L, Ghilardi L, Arnoldi E, Tondini CA, Bettini AC. A rapid fatal evolution of coronavirus disease-19 in a patient with advanced lung cancer with a long-time response to Nivolumab. J Thorac Oncol. 2020;15:e83–e5.
Lovly CM, Boyd KL, Gonzalez-Ericsson PI, Lowe CL, Brown HM, Hoffman RD, et al. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv. 2020.
Murata D, Azuma K, Tokisawa S, Tokito T, Hoshino T. A case of cytokine release syndrome accompanied with COVID-19 infection during treatment with immune checkpoint inhibitors for non-small cell lung cancer. Thorac Cancer. 2022;13:2911–4.
Nishiyama K, Morikawa K, Shinozaki Y, Ueno J, Tanaka S, Tsuruoka H, et al. Case report: Electrocardiographic changes in pembrolizumab-induced fatal myocarditis. Front Immunol. 2023;14:1078838.
Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27:1362–6.
Blaise M, Rocher F, Spittler H, Sanchez A, Lanteri E, Coco L, et al. Severe necrotizing myopathy after COVID-19 vaccine with BNT162b2 and regimen with ipilimumab plus nivolumab in a patient with advanced melanoma. J Eur Acad Dermatol Venereol. 2022;36:e100–e2.
Chong K-M, Yang C-Y, Lin C-C, Lien W-C. Severe immune thrombocytopenia following COVID-19 vaccination (Moderna) and immune checkpoint inhibitor. Am J Emerg Med. 2022;56:395.e1-.e3.
El-Behaedi S, Ng S, Goyal PK, Choi JN. Widespread cutaneous eruption following COVID-19 vaccine in the setting of immunotherapy. JAAD Case Rep. 2022;29:48–50.
Hussain K, Kawsar A, Weir J, Au L, Turajlic S, Larkin J, et al. Severe cutaneous adverse reaction following COVID-19 vaccination and immunotherapy: a second hit? Clin Exp Dermatol. 2022;47:149–51.
Lasagna A, Lenti MV, Cassaniti I, Sacchi P. Development of hepatitis triggered by SARS-CoV-2 vaccination in patient with cancer during immunotherapy: a case report. Immunotherapy 2022;14:915–25.
Makiguchi T, Fukushima T, Tanaka H, Taima K, Takayasu S, Tasaka S. Diabetic ketoacidosis shortly after COVID-19 vaccination in a non-small-cell lung cancer patient receiving combination of PD-1 and CTLA-4 inhibitors: a case report. Thorac Cancer. 2022;13:1220–3.
Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol Ther. 2021;34:e15055.
Ohuchi K, Amagai R, Tamabuchi E, Kambayashi Y, Fujimura T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J Dermatol. 2022;49:e167–e8.
Sato T, Kodama S, Kaneko K, Imai J, Katagiri H. Type 1 Diabetes Mellitus associated with nivolumab after second SARS-CoV-2 vaccination, Japan. Emerg Infect Dis. 2022;28:1518–20.
Sumi T, Koshino Y, Michimata H, Nagayama D, Watanabe H, Yamada Y, et al. Cytokine release syndrome in a patient with non-small cell lung cancer on ipilimumab and nivolumab maintenance therapy after vaccination with the mRNA-1273 vaccine: a case report. Transl Lung Cancer Res. 2022;11:1973–6.
Takenaka M, Nakamori M, Ishikawa R, Aoki S, Maruyama H. Encephalopathy after COVID-19 vaccination during treatment with nivolumab: a case report. Clin Neurol Neurosurg. 2023;226:107632.
Tripathy S, Alvarez N, Jaiswal S, Williams R, Al-Khadimi M, Hackman S, et al. Hypermetabolic lymphadenopathy following the administration of COVID-19 vaccine and immunotherapy in a lung cancer patient: a case report. J Med Case Rep. 2022;16:445.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7.
Moritz RKC, Gutzmer R, Zimmer L, Meier F, Ahmed MS, Sell S, et al. SARS-CoV-2 infections in melanoma patients treated with PD-1 inhibitors: a survey of the German ADOREG melanoma registry. Eur J Cancer. 2021;144:382–5.
Bersanelli M, Scala S, Affanni P, Veronesi L, Colucci ME, Banna GL, et al. Immunological insights on influenza infection and vaccination during immune checkpoint blockade in cancer patients. Immunotherapy 2020;12:105–10.
Burgaletto C, Brunetti O, Munafo A, Bernardini R, Silvestris N, Cantarella G. et al. Lights and shadows on managing immune checkpoint inhibitors in oncology during the COVID-19 Era. Cancers (Basel). 2021;13:1906.
Yatim N, Boussier J, Tetu P, Smith N, Bruel T, Charbit B, et al. Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection. Sci Adv. 2021;7:eabg4081.
Chiappelli F, Khakshooy A, Greenberg G. CoViD-19 Immunopathology and Immunotherapy. Bioinformation 2020;16:219–22.
Godel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018;44:371–3.
Biondillo DE, Konicek SA, Iwamoto GK. Interferon-gamma regulation of interleukin 6 in monocytic cells. Am J Physiol. 1994;267:L564–8.
Garassino MC, Ribas A. At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol Res. 2021;9:261–4.
Lazarus G, Budiman RA, Rinaldi I. Does immune checkpoint inhibitor increase the risks of poor outcomes in COVID-19-infected cancer patients? A systematic review and meta-analysis. Cancer Immunol Immunother. 2022;71:373–86.
Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharm. 2020;11:557.
Guo M, Liu J, Miao R, Ahmed Z, Yu J, Guan J, et al. A single center retrospective study of the impact of COVID-19 infection on immune-related adverse events in cancer patients receiving immune checkpoint inhibitors. J Immunother. 2022;45:389–95.
Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–7.
Wankhede D, Grover S, Hofman P. Determinants of humoral immune response to SARS-CoV-2 vaccines in solid cancer patients: a systematic review and meta-analysis. Vaccine 2023;41:1791–8.
Ruiz JI, Lopez-Olivo MA, Geng Y, Suarez-Almazor ME. COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother Cancer. 2023;11:e006246.
Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681–91.
Kian W, Zemel M, Kestenbaum EH, Rouvinov K, Alguayn W, Levitas D, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: a retrospective single-center study. Med (Baltim). 2022;101:e28561.
Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–3.
Mei Q, Hu G, Yang Y, Liu B, Yin J, Li M, et al. Impact of COVID-19 vaccination on the use of PD-1 inhibitor in treating patients with cancer: a real-world study. J Immunother Cancer. 2022;10:e004157.
Strobel SB, Machiraju D, Kalber KA, Hassel JC. Immune-related adverse events of COVID-19 vaccination in skin cancer patients receiving immune-checkpoint inhibitor treatment. Cancer Immunol Immunother. 2022;71:2051–6.
Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022;165:386–401.
Walle T, Bajaj S, Kraske JA, Rosner T, Cussigh CS, Kalber KA, et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer. 2022;3:1039–51.
Baden MY, Imagawa A, Abiru N, Awata T, Ikegami H, Uchigata Y, et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int. 2019;10:58–66.
Pietropaolo M, Hotez P, Giannoukakis N. Incidence of an insulin-requiring hyperglycemic syndrome in SARS-CoV-2-infected young individuals: is it type 1 diabetes? Diabetes 2022;71:2656–63.
Ben Nasr M, D'Addio F, Montefusco L, Usuelli V, Loretelli C, Rossi A, et al. Indirect and direct effects of SARS-CoV-2 on human pancreatic islets. Diabetes 2022;71:1579–90.
Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K. Acute hyperglycaemic crisis after vaccination against COVID-19: a case series. Diabet Med. 2021;38:e14631.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines: a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81970583 and 82060138), the Kidney Disease Engineering Technology Research Centre Foundation of Jiangxi Province (No. 20164BCD40095), the Key Project of Jiangxi Provincial Nature Science Foundation (No. 20224ACB206008), and the “Thousand Talents Plan” project of introducing and training high-level talents of innovation and entrepreneurship in Jiangxi Province (No. JXSQ2023201030).
Author information
Authors and Affiliations
Contributions
YY conducted data collection, analysis, wrote original draft and revise draft. GX was responsible for ideas, funds, and paper revision. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Hans-Uwe Simon
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Xu, G. SARS-CoV-2 infection and COVID-19 vaccination in cancer patients undergoing immune checkpoint inhibitors. Cell Death Dis 14, 390 (2023). https://doi.org/10.1038/s41419-023-05922-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05922-w