Abstract
Background
KRAS is one of the most frequently mutated oncogenes in various cancers, and several novel KRAS G12C direct inhibitors are now in clinical trials. Here, we characterised the anti-tumour efficacy of ASP2453, a novel KRAS G12C inhibitor, in preclinical models of KRAS G12C-mutated cancer.
Methods
We evaluated the in vitro and in vivo activity of ASP2453, alone or in combination with targeted agents and immune checkpoint inhibitors, in KRAS G12C-mutated cancer cells and xenograft models. We also assessed pharmacological differences between ASP2453 and AMG 510, another KRAS G12C inhibitor, using an SPR assay, washout experiments and an AMG 510-resistant xenograft model.
Results
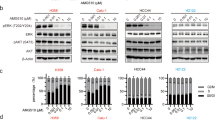
ASP2453 potently and selectively inhibited KRAS G12C-mediated growth, KRAS activation and downstream signalling in vitro and in vivo, and improved the anti-tumour effects of targeted agents and immune checkpoint inhibitors. Further, ASP2453 had more rapid binding kinetics to KRAS G12C protein and showed more potent inhibitory effects on KRAS activation and cell proliferation after washout than AMG 510. ASP2453 also induced tumour regression in an AMG 510-resistant xenograft model.
Conclusions
ASP2453 is a potential therapeutic agent for KRAS G12C-mutated cancer. ASP2453 showed efficacy in AMG 510-resistant tumours, even among compounds with the same mode of action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6.
Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018;15:709–20.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–74.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13:828–51.
Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res. 2015;13:1325–35.
Guerrero S, Casanova I, Farré L, Mazo A, Capellà G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6.
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Ahrendt SA, Decker PA, Alawi EA, Zhu Y-r, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–30.
Dogan S, Shen R, Ang DC, Johnson ML, D’Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–77.
Nadal E, Chen G, Prensner JR, Shiratsuchi H, Sam C, Zhao L, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. 2014;9:1513–22.
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–51.
Lim SM, Westover KD, Ficarro SB, Harrison RA, Choi HG, Pacold ME, et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew Chem Int Ed Engl. 2014;53:199–204.
Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–23.
Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63:52–65.
Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. The KRAS(G12C) inhibitor MRTX849 provides Insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Disco. 2020;10:54–71.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl J Med. 2020;383:1207–17.
Jänne, PA, Rybkin, II, Spira, AI, Riely, GJ, Papadopoulos, KP, Sabari, JK, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in advanced/metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Presented at the 2020 AACR-NCI-EORTC meeting (2020).
Johnson, ML, Ou, SHI, Barve, M, Rybkin, II, Papadopoulos, KP, Leal, TA, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Presented at the 2020 AACR-NCI-EORTC meeting (2020).
Kim D, Xue JY, Lito P. Targeting KRAS(G12C): from inhibitory mechanism to modulation of antitumor effects in patients. Cell. 2020;183:850–9.
Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172:578–89. e517
Patricelli MP, Janes MR, Li LS, Hansen R, Peters U, Kessler LV, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6:316–29.
Molina-Arcas M, Moore C, Rana S, van Maldegem F, Mugarza E, Romero-Clavijo P, et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci Transl Med. 2019;11:eaaw7999.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Tiwary P, Limongelli V, Salvalaglio M, Parrinello M. Kinetics of protein–ligand unbinding: predicting pathways, rates, and rate-limiting steps. Proc Natl Acad Sci USA. 2015;112:E386–91.
Copeland RA. The drug–target residence time model: a 10-year retrospective. Nat Rev Drug Discov. 2016;15:87–95.
Palkowitz MD, Tan B, Hu H, Roth K, Bauer RA. Synthesis of diverse N-acryloyl azetidines and evaluation of their enhanced thiol reactivities. Org Lett. 2017;19:2270–3.
Acknowledgements
We thank Dr Hirofumi Ishii, Dr Yukinori Shimoshige and Mr Yosuke Yamanaka for support with the biological study. We are grateful to Dr Masahiko Hayakawa and Dr Taku Yoshida for their useful suggestions.
Funding
This study was funded by Astellas Pharma Inc.
Author information
Authors and Affiliations
Contributions
AN, TN and MS conceived the study and designed experiments. AN, YN, TN, MS, KK, KM, KH and MY developed methodology. AN, YN, TN, KK, KM, KH and MY acquired and analysed the data. All authors edited the manuscript and approved the submission of the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No human participants, human data or human tissue were used as parts of this study. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc., Tsukuba Research Center, which is accredited by AAALAC International.
Consent for publication
Not applicable.
Competing interests
All authors are employees of Astellas Pharma Inc. and its affiliates.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nakayama, A., Nagashima, T., Nishizono, Y. et al. Characterisation of a novel KRAS G12C inhibitor ASP2453 that shows potent anti-tumour activity in KRAS G12C-mutated preclinical models. Br J Cancer 126, 744–753 (2022). https://doi.org/10.1038/s41416-021-01629-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01629-x