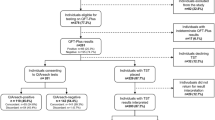
Abstract
Tuberculosis (TB) is a major infectious complication in hematopoietic stem cell transplant (HSCT) recipients in countries with high TB prevalence. Identifying and treating latent tuberculosis infection (LTBI) helps to prevent TB reactivation after transplantation. Few studies have compared the tuberculin skin test (TST) with interferon Gamma release assays (IGRA) to diagnose LTBI in HSCT candidates. We compared TST and QuantiFeron TB gold in tube (QTF-GIT) and prospectively evaluated the incidence of active tuberculosis in 126 HSCT candidates and 58 HSCT recipients with chronic GVHD followed at the outpatient clinic. TB was diagnosed by culture in Mycobacteria media and by commercial real-time PCR kit. Considering the positivity of any test, the prevalence of LTBI was 8.7% in HSCT candidates (11 out of 126) and 12.5% in HSCT recipients with chronic GVHD (6 out of 48). QTF-GIT indeterminate results were detected in 2.4% of the HSCT candidates. Fair to good agreement (K > 0.50) between tests was observed in both cohorts. Cumulative incidence of TB was 3% in the GVHD cohort. TB was diagnosed in 2 chronic GVHD recipients, both cases confirmed by positive culture and PCR. None of the 11 patients with LTBI diagnosed pre-HSCT who received INH prophylaxis developed TB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
WHO. The top 10 causes of death. 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
World Health Organization (WHO). Global Tuberculosis Report 2020. World Health Organization (WHO): Geneva: WHO press; 2020. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf.
World Health Organization (WHO). Global Tuberculosis Report 2018. Geneve: World Health Organization (WHO); 2018. pp. 1–265. https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1.
Ministéro da Saúde. Overview of tuberculosis in Brazil: epidemiological and operational indicators. Coordenação-Geral de Documentação e Informação. 2020. http://www.aids.gov.br/pt-br/pub/2019/panorama-da-tuberculose-no-brasil-indicadores-epidemiologicos-e-operacionais.
Köseoğlu F, Emiroğlu R, Karakayali H, Bilgin N, Haberal M. Prevalence of mycobacterial infection in solid organ transplant recipients. Transpl Proc. 2001;33:1782–4.
Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG. et al. The risk of tuberculosis in transplant candidates and recipients: A TBNET consensus statement. Eur Respir J. 2012;40:990–1013.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transpl. 2009;15:1143–238.
Munoz L, Gomila A, Casas S, Castellote J, Arnan M, Rafecas A, et al. Immunodiagnostic tests’ predictive values for progression to tuberculosis in transplant recipients: a prospective cohort study. Transpl Direct. 2015;1:e12.
Kim S-H, Lee S-O, Park JB, Park I-A, Park SJ, Yun S-C, et al. A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am J Transpl. 2011;11:1927–35.
Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transpl. 2007;7:2797–801.
Moon SM, Lee SO, Choi SH, Kim YS, Woo JH, Yoon DH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis. 2013;15:104–9.
Moon H-W, Hur M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection: an updated review. Ann Clin Lab Sci. 2013;43:221–9.
Seyhan EC, Sökücü S, Altin S, Günlüoǧlu G, Trablus S, Yilmaz D, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis. 2010;12:98–105.
Lee JE, Kim H-J, Lee SW. The clinical utility of tuberculin skin test and interferon-γ release assay in the diagnosis of active tuberculosis among young adults: a prospective observational study. BMC Infect Dis. 2011;11:96.
Lee YM, Lee SO, Choi SH, Kim YS, Woo JH, Kim DY, et al. A prospective longitudinal study evaluating the usefulness of the interferon-gamma releasing assay for predicting active tuberculosis in allogeneic hematopoietic stem cell transplant recipients. J Infect. 2014;69:165–73.
Rahimifard N, Mahmoudi S, Mamishi S, Pourakbari B. Prevalence of latent tuberculosis infection in transplant candidates: A systematic review and meta-analysis. Micro Pathog. 2018;125:401–10.
Qin L, Wang Q-R, Wang Q, Yao H, Wen L, Wu L, et al. T-SPOT.TB for Detection of Tuberculosis Infection among Hematological Malignancy Patients and Hematopoietic Stem Cell Transplant Recipients. Asian Pacific. J Cancer Prev. 2013;14:7415–9.
ECIL-8. Latent TB infection (LTBI) and active TB infection [Internet]. 8th European Conference in Infections in Leukemia (ECIL-8). Sophia Antipolis, France; 2019. pp. 1–30. http://www.ecil-leukaemia.com/resources/Tuberculosis and atypical mycobacterial infections - Final Slide Set.pdf.
Acknowledgements
The study received financial support from FAPESP grant 2010/08816–1. The authors thank Giovana H. da Rocha, Anderson João Simioni and Ana Paula Ribeiro for database update support, and the Centro de Laboratórios Regionais (Núcleo de Ciências Biomédicas) - Instituto Adolfo Lutz Bauru II for performing the culture in Mycobacteria media during the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Rodrigues, M., de Almeida Testa, L.H., dos Santos, A.C.F. et al. Latent and active tuberculosis infection in allogeneic hematopoietic stem cell transplant recipients: a prospective cohort study. Bone Marrow Transplant 56, 2241–2247 (2021). https://doi.org/10.1038/s41409-021-01329-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01329-3
This article is cited by
-
Prevalence of latent Mycobacterium tuberculosis infection in hematopoietic stem cell transplantation comparing tuberculin skin test and interferon-gamma release assay
European Journal of Clinical Microbiology & Infectious Diseases (2023)