Abstract
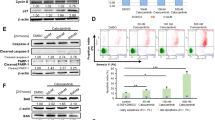
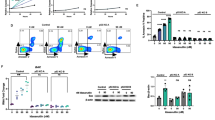
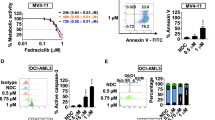
Understanding the molecular pathogenesis of acute myeloid leukemia (AML) with well-defined genomic abnormalities has facilitated the development of targeted therapeutics. Patients with t(8;21) AML frequently harbor a fusion gene RUNX1-RUNX1T1 and KIT mutations as “secondary hit”, making the disease one of the ideal models for exploring targeted treatment options in AML. In this study we investigated the combination therapy of agents targeting RUNX1-RUNX1T1 and KIT in the treatment of t(8;21) AML with KIT mutations. We showed that the combination of eriocalyxin B (EriB) and homoharringtonine (HHT) exerted synergistic therapeutic effects by dual inhibition of RUNX1-RUNX1T1 and KIT proteins in Kasumi-1 and SKNO-1 cells in vitro. In Kasumi-1 cells, the combination of EriB and HHT could perturb the RUNX1-RUNX1T1-responsible transcriptional network by destabilizing RUNX1-RUNX1T1 transcription factor complex (AETFC), forcing RUNX1-RUNX1T1 leaving from the chromatin, triggering cell cycle arrest and apoptosis. Meanwhile, EriB combined with HHT activated JNK signaling, resulting in the eventual degradation of RUNX1-RUNX1T1 by caspase-3. In addition, HHT and EriB inhibited NF-κB pathway through blocking p65 nuclear translocation in two different manners, to synergistically interfere with the transcription of KIT. In mice co-expressing RUNX1-RUNX1T1 and KITN822K, co-administration of EriB and HHT significantly prolonged survival of the mice by targeting CD34+CD38− leukemic cells. The synergistic effects of the two drugs were also observed in bone marrow mononuclear cells (BMMCs) of t(8;21) AML patients. Collectively, this study reveals the synergistic mechanism of the combination regimen of EriB and HHT in t(8;21) AML, providing new insight into optimizing targeted treatment of AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Thol F. What to use to treat AML: the role of emerging therapies. Hematology. 2021;2021:16–23.
Döhner H, Wei AH, Löwenberg B. Towards precision medicine for AML. Nat Rev Clin Oncol. 2021;18:577–90.
Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 2021;19:16–27.
Elagib KE, Goldfarb AN. Oncogenic pathways of AML1-ETO in acute myeloid leukemia: multifaceted manipulation of marrow maturation. Cancer Lett. 2007;251:179–86.
Hollein A, Nadarajah N, Meggendorfer M, Jeromin S, Kern W, Haferlach C, et al. Molecular characterization of AML with RUNX1-RUNX1T1 at diagnosis and relapse reveals net loss of co-mutations. Hemasphere. 2019;3:e178.
Wang J, Hoshino T, Redner RL, Kajigaya S, Liu JM. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci USA. 1998;95:10860–5.
Amann JM, Nip J, Strom DK, Lutterbach B, Harada H, Lenny N, et al. ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol Cell Biol. 2001;21:6470–83.
Trombly DJ, Whitfield TW, Padmanabhan S, Gordon JA, Lian JB, van Wijnen AJ, et al. Genome-wide co-occupancy of AML1-ETO and N-CoR defines the t(8;21) AML signature in leukemic cells. BMC Genomics. 2015;16:309.
Wang L, Gural A, Sun XJ, Zhao X, Perna F, Huang G, et al. The leukemogenicity of AML1-ETO is dependent on site-specific lysine acetylation. Science. 2011;333:765–9.
Li Y, Wang H, Wang X, Jin W, Tan Y, Fang H, et al. Genome-wide studies identify a novel interplay between AML1 and AML1/ETO in t(8;21) acute myeloid leukemia. Blood. 2016;127:233–42.
Ptasinska A, Pickin A, Assi SA, Chin PS, Ames L, Avellino R, et al. RUNX1-ETO depletion in t(8;21) AML leads to C/EBPalpha- and AP-1-mediated alterations in enhancer-promoter interaction. Cell Rep. 2019;28:3022–31.
Sun XJ, Wang Z, Wang L, Jiang Y, Kost N, Soong TD, et al. A stable transcription factor complex nucleated by oligomeric AML1-ETO controls leukaemogenesis. Nature. 2013;500:93–7.
Duployez N, Marceau-Renaut A, Boissel N, Petit A, Bucci M, Geffroy S, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood. 2016;127:2451–9.
Wichmann C, Quagliano-Lo Coco I, Yildiz O, Chen-Wichmann L, Weber H, Syzonenko T, et al. Activating c-KIT mutations confer oncogenic cooperativity and rescue RUNX1/ETO-induced DNA damage and apoptosis in human primary CD34+ hematopoietic progenitors. Leukemia. 2015;29:279–89.
Yu X, Ruan X, Zhang J, Zhao Q. Celastrol induces cell apoptosis and inhibits the expression of the AML1-ETO/C-KIT oncoprotein in t(8;21) leukemia. Molecules. 2016;21:574.
Hui H, Zhang X, Li H, Liu X, Shen L, Zhu Y, et al. Oroxylin A, a natural anticancer flavonoid compound, induces differentiation of t(8;21)-positive Kasumi-1 and primary acute myeloid leukemia cells. J Cancer Res Clin Oncol. 2016;142:1449–59.
Sawney S, Arora R, Aggarwal KK, Saluja D. Esculetin downregulates the expression of AML1-ETO and C-KIT in Kasumi-1 cell line by decreasing half-life of mRNA. J Oncol. 2015;2015:781473.
Oo ZM, Illendula A, Grembecka J, Schmidt C, Zhou Y, Esain V, et al. A tool compound targeting the core binding factor Runt domain to disrupt binding to CBFbeta in leukemic cells. Leuk Lymphoma. 2018;59:2188–200.
Paschka P, Schlenk RF, Weber D, Benner A, Bullinger L, Heuser M, et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia. 2018;32:1621–30.
Zhen T, Wu CF, Liu P, Wu HY, Zhou GB, Lu Y, et al. Targeting of AML1-ETO in t(8;21) leukemia by oridonin generates a tumor suppressor-like protein. Sci Transl Med. 2012;4:127ra38.
Wang L, Zhao WL, Yan JS, Liu P, Sun HP, Zhou GB, et al. Eriocalyxin B induces apoptosis of t(8;21) leukemia cells through NF-kappaB and MAPK signaling pathways and triggers degradation of AML1-ETO oncoprotein in a caspase-3-dependent manner. Cell Death Differ. 2007;14:306–17.
Chen XJ, Zhang WN, Chen B, Xi WD, Lu Y, Huang JY, et al. Homoharringtonine deregulates MYC transcriptional expression by directly binding NF-kappaB repressing factor. Proc Natl Acad Sci USA. 2019;116:2220–5.
Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–15.
Wang YY, Zhao LJ, Wu CF, Liu P, Shi L, Liang Y, et al. C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci USA. 2011;108:2450–5.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81.
Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51.
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D12.
Liu S, Wu LC, Pang J, Santhanam R, Schwind S, Wu YZ, et al. Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell. 2010;17:333–47.
Goyama S, Mulloy JC. Molecular pathogenesis of core binding factor leukemia: current knowledge and future prospects. Int J Hematol. 2011;94:126–33.
Ptasinska A, Assi Salam A, Martinez-Soria N, Imperato Maria R, Piper J, Cauchy P, et al. Identification of a dynamic core transcriptional network in t(8;21) AML that regulates differentiation block and self-renewal. Cell Rep. 2014;8:1974–88.
Mangaonkar AA, Patnaik MM. Hereditary predisposition to hematopoietic neoplasms: when bloodline matters for blood cancers. Mayo Clin Proc. 2020;95:1482–98.
Kato H, Igarashi K. To be red or white: lineage commitment and maintenance of the hematopoietic system by the “inner myeloid”. Haematologica. 2019;104:1919–27.
Lieu YK, Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci USA. 2009;106:21689–94.
Nafria M, Keane P, Ng ES, Stanley EG, Elefanty AG, Bonifer C. Expression of RUNX1-ETO rapidly alters the chromatin landscape and growth of early human myeloid precursor cells. Cell Rep. 2020;31:107691.
Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK: an intricate affair. Cell Cycle. 2004;3:1524–9.
Rovida E, Gozzini A, Barbetti V, Giuntoli S, Santini V, Dello Sbarba P. The c-Jun-N-terminal-Kinase inhibitor SP600125 enhances the butyrate derivative D1-induced apoptosis via caspase 8 activation in Kasumi 1 t(8;21) acute myeloid leukaemia cells. Br J Haematol. 2006;135:653–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81670137), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152501), and State Key Laboratory of Medical Genomics Support (201802), and Jiading District Health Commission Chinese Medicine Youth Research Support (2019-QN-ZYY-03).
Author information
Authors and Affiliations
Contributions
BC conceived and supervised the study. YCL and XJC conducted the main experiments. BD, JYW, and MHL participated in the experiments. YTD performed RNA-seq and ChIP-seq analysis. PL, HL, and KKW gave the technical support. BC, XJC, YCL, and LJ wrote the paper. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, Yc., Chen, Xj., Dai, Yt. et al. Combination of eriocalyxin B and homoharringtonine exerts synergistic anti-tumor effects against t(8;21) AML. Acta Pharmacol Sin 45, 633–645 (2024). https://doi.org/10.1038/s41401-023-01196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01196-2