Abstract
Background
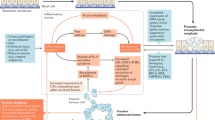
Inflammatory bowel disease (IBD) has been implicated as a risk factor for prostate cancer, however, the mechanism of how IBD leads to prostate tumorigenesis is not known. Here, we investigated whether chronic intestinal inflammation leads to pro-inflammatory changes associated with tumorigenesis in the prostate.
Methods
Using clinical samples of men with IBD who underwent prostatectomy, we analyzed whether prostate tumors had differences in lymphocyte infiltrate compared to non-IBD controls. In a mouse model of chemically-induced intestinal inflammation, we investigated whether chronic intestinal inflammation could be transferred to the wild-type mouse prostate. In addition, mouse prostates were evaluated for activation of pro-oncogenic signaling and genomic instability.
Results
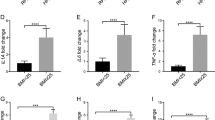
A higher proportion of men with IBD had T and B lymphocyte infiltration within prostate tumors. Mice with chronic colitis showed significant increases in prostatic CD45 + leukocyte infiltration and elevation of three pro-inflammatory cytokines—TIMP-1, CCL5, and CXCL1 and activation of AKT and NF-kB signaling pathways. Lastly, mice with chronic colitis had greater prostatic oxidative stress/DNA damage, and prostate epithelial cells had undergone cell cycle arrest.
Conclusions
These data suggest chronic intestinal inflammation is associated with an inflammatory-rich, pro-tumorigenic prostatic phenotype which may explain how gut inflammation fosters prostate cancer development in men with IBD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
22 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41391-021-00409-1
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. 2020;70:7–30.
Hugosson J, Roobol MJ, Månsson M, Tammela TLJ, Zappa M, Nelen V, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur Urol. 2019;76:43–51.
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–55.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35.
Force UPST. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319:1901–13.
Burns JA, Weiner AB, Catalona WJ, Li EV, Schaeffer EM, Hanauer SB. et al. Inflammatory bowel disease and the risk of prostate cancer. Eur Urol. 2019;75:846–52.
Meyers TJ, Weiner AB, Graff RE, Desai AS, Cooley LF, Catalona WJ, et al. Association between inflammatory bowel disease and prostate cancer: a large-scale, prospective, population-based study. Int J Cancer. 2020:2020.01.16.20017707.
Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease—United States, 2015 and 2016. MMWR Morbidity Mortal Wkly Rep. 2018;67:190–5.
Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441–52.
Waldner MJ, Neurath MF. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1:6–16.
De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92.
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–309.
Kitajima S, Takuma S, Morimoto M. Tissue distribution of dextran sulfate sodium (DSS) in the acute phase of murine DSS-induced colitis. J Vet Med Sci. 1999;61:67–70.
Hnatyszyn A, Hryhorowicz S, Kaczmarek-Ryś M, Lis E, Słomski R, Scott RJ, et al. Colorectal carcinoma in the course of inflammatory bowel diseases. Hereditary cancer Clin Pract. 2019;17:18.
Song G, Xu S, Zhang H, Wang Y, Xiao C, Jiang T, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35:148.
Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376.
Kuo PL, Shen KH, Hung SH, Hsu YL. CXCL1/GROα increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic regulation. Carcinogenesis. 2012;33:2477–87.
Höfner T, Klein C, Eisen C, Rigo-Watermeier T, Haferkamp A, Trumpp A, et al. 147 The C-Myc and TNFα/NF-kB pathways are critically involved in the regulatory network between the undifferentiated prostate basal stem cell state and the more differentiated luminal prostate epithelial cells. Eur Urol Suppl. 2016;15:e147.
Clegg NJ, Couto SS, Wongvipat J, Hieronymus H, Carver BS, Taylor BS, et al. MYC Cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PloS ONE. 2011;6:e17449.
Frick A, Khare V, Paul G, Lang M, Ferk F, Knasmüller S, et al. Overt increase of oxidative stress and DNA damage in murine and human colitis and colitis-associated neoplasia. Mol Cancer Res. 2018;16:634–42.
Breitzig M, Bhimineni C, Lockey R, Kolliputi N. 4-Hydroxy-2-nonenal: a critical target in oxidative stress? Am J Physiol Cell Physiol. 2016;311:C537–43.
Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71:1669–79.
Lawless C, Wang C, Jurk D, Merz A, Zglinicki T, Passos JF. Quantitative assessment of markers for cell senescence. Exp Gerontol. 2010;45:772–8.
Garraud O, Borhis G, Badr G, Degrelle S, Pozzetto B, Cognasse F, et al. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond. BMC Immunol. 2012;13:63.
Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016–29.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–84.
Liou GY. Inflammatory cytokine signaling during development of pancreatic and prostate cancers. J Immunol Res. 2017;2017:7979637.
Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020;12:287.
Viennois E, Chen F, Merlin D. NF-κB pathway in colitis-associated cancers. Transl Gastrointest Cancer. 2013;2:21–9.
Tang F, Wang Y, Hemmings BA, Rüegg C, Xue G. PKB/Akt-dependent regulation of inflammation in cancer. Semin Cancer Biol. 2018;48:62–9.
Zeng S, Shen WH, Liu L. Senescence and cancer. Cancer Transl Med. 2018;4:70–4.
Massari F, Mollica V, Di Nunno V, Gatto L, Santoni M, Scarpelli M, et al. The Human Microbiota and Prostate Cancer: Friend or Foe? Cancers. 2019;11:459.
Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794–801.
Acknowledgements
This work was supported by the SPORE in Prostate Cancer (P50 CA180995) (JW, SAA, SDK). The project described was supported by the Robert H. Lurie Comprehensive Cancer Center, Northwestern University. We thank Northwestern University Histology and Phenotyping Core Laboratory for technical support which is supported by NCI P30-CA060553.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to missing references.
Rights and permissions
About this article
Cite this article
Desai, A.S., Sagar, V., Lysy, B. et al. Inflammatory bowel disease induces inflammatory and pre-neoplastic changes in the prostate. Prostate Cancer Prostatic Dis 25, 463–471 (2022). https://doi.org/10.1038/s41391-021-00392-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00392-7
This article is cited by
-
Association between inflammatory bowel disease and risk of incident prostate cancer: a systematic review and meta-analysis of cohort studies
International Journal of Colorectal Disease (2023)