Abstract
Background
Osteogenesis imperfecta (OI) is associated with short stature, which is mild, severe and moderate in OI types I, III and IV, respectively. Standardized OI type- and sex-specific growth charts across all pediatric ages do not exist.
Methods
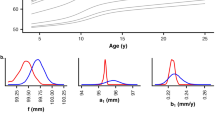
We assessed 573 individuals with OI (type I, III or IV), each with at least one height measurement between ages 3 months and 20 years (total 6523 observations). Analogous to the Centers for Disease Control pediatric growth charts, we generated OI type- and sex-specific growth charts for infants (ages 3–36 months) as well as children and adolescents (ages 2–20 years). Growth curves were fitted to the data using the LMS method and percentiles were smoothed.
Results
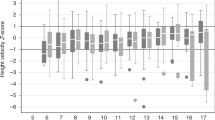
Age was associated with a decline in height z-scores (p < 0.001 for all OI types), which was more pronounced in females. Height multiplier curves were produced to predict adult height in children with OI. Among individuals with OI type I, those with COL1A1 pathogenic variants leading to haploinsufficiency were taller than those with COL1A1 or COL1A2 pathogenic variants not leading to haploinsufficiency.
Conclusion
Our standardized OI type- and sex-specific growth charts can be used to assess the growth of individuals with OI from infancy to adulthood.
Impact
-
Standardized osteogenesis imperfecta (OI) type- and sex-specific growth charts across all pediatric ages do not exist.
-
Our study is the first to generate OI type- and sex-specific growth charts across all pediatric ages.
-
Our height multiplier curves can be utilized to predict adult height in children with OI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy and ethical concerns, but are available from the corresponding author on reasonable request.
References
Forlino, A. & Marini, J. C. Osteogenesis imperfecta. Lancet 387, 1657–1671 (2016).
Robinson, M.-E. & Rauch, F. Mendelian bone fragility bisorders. Bone 126, 11–17 (2019).
Trejo, P. & Rauch, F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos. Int. 27, 3427–3437 (2016).
Jain, M. et al. Growth characteristics in individuals with osteogenesis imperfecta in North America: results from a multicenter study. Genet Med. 21, 275–283 (2019).
Marr, C., Seasman, A. & Bishop, N. Managing the patient with osteogenesis imperfecta: a multidisciplinary approach. J. Multidiscip. Healthc. 10, 145–155 (2017).
Robinson, M. E., Rauch, D., Glorieux, F. H. & Rauch, F. Pubertal growth in osteogenesis imperfecta caused by pathogenic variants in Col1a1/Col1a2. Genet Med. 24, 1920–1926 (2022).
Graff, K. & Syczewska, M. Developmental charts for children with osteogenesis imperfecta, type I (body height, body weight and BMI). Eur. J. Pediatr. 176, 311–316 (2017).
Barber, L. A. et al. Longitudinal growth curves for children with classical osteogenesis imperfecta (types III and IV) caused by structural pathogenic variants in type I collagen. Genet Med. 21, 1233–1239 (2019).
Paley, J. et al. The multiplier method for prediction of adult height. J. Pediatr. Orthop. 24, 732–737 (2004).
Paley, D., Matz, A. L., Kurland, D. B., Lamm, B. M. & Herzenberg, J. E. Multiplier method for prediction of adult height in patients with achondroplasia. J. Pediatr. Orthop. 25, 539–542 (2005).
Mortier, G. R. et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet A 179, 2393–2419 (2019).
Bardai, G., Moffatt, P., Glorieux, F. H. & Rauch, F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta: diagnostic yield and mutation spectrum. Osteoporos. Int. 27, 3607–3613 (2016).
Montpetit, K., Palomo, T., Glorieux, F. H., Fassier, F. & Rauch, F. Multidisciplinary treatment of severe osteogenesis imperfecta: functional outcomes at skeletal maturity. Arch. Phys. Med. Rehabil. 96, 1834–1839 (2015).
Robinson, M. E., Trejo, P., Palomo, T., Glorieux, F. H. & Rauch, F. Osteogenesis imperfecta: skeletal outcomes after bisphosphonate discontinuation at final height. J. Bone Miner. Res. 34, 2198–2204 (2019).
Fassier, F. Fassier-Duval telescopic system: how I do it? J. Pediatr. Orthop. 37, S48–S51 (2017).
Ogden, C. L. et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics Version. Pediatrics 109, 45–60 (2002).
Cole, T. J. Fitting smoothed centile curves to reference data. J. R. Stat. Soc. Ser. A (Stat. Soc.) 151, 385–418 (1988).
Butler, M. G. et al. Growth charts for non-growth hormone treated Prader-Willi syndrome. Pediatrics 135, e126–e135 (2015).
Cole, T. J. & Green, P. J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat. Med. 11, 1305–1319 (1992).
Germain-Lee, E. L. et al. Cross-sectional and longitudinal growth patterns in osteogenesis imperfecta: implications for clinical care. Pediatr. Res. 79, 489–495 (2016).
Zemel, B. S. et al. Growth charts for children with Down syndrome in the United States. Pediatrics 136, e1204–e1211 (2015).
Horton, W. A., Rotter, J. I., Rimoin, D. L., Scott, C. I. & Hall, J. G. Standard growth curves for achondroplasia. J. Pediatr. 93, 435–438 (1978).
Patel, P. et al. Growth charts for patients with Hunter syndrome. Mol. Genet Metab. Rep. 1, 5–18 (2014).
Muschol, N. M. et al. Growth charts for patients with Sanfilippo syndrome (Mucopolysaccharidosis type III). Orphanet J. Rare Dis. 14, 93 (2019).
Montano, A. M., Tomatsu, S., Brusius, A., Smith, M. & Orii, T. Growth charts for patients affected with Morquio A disease. Am. J. Med. Genet A 146A, 1286–1295 (2008).
Quartel, A. et al. Growth charts for individuals with Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). JIMD Rep. 18, 1–11 (2015).
Ben Amor, I. M., Roughley, P., Glorieux, F. H. & Rauch, F. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in Col1a1. J. Bone Min. Res. 28, 2001–2007 (2013).
Ma, J. et al. Impact of vertebral fractures and glucocorticoid exposure on height deficits in children during treatment of leukemia. J. Clin. Endocrinol. Metab. 104, 213–222 (2019).
Rauch, F., Lalic, L., Roughley, P. & Glorieux, F. H. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J. Bone Min. Res. 25, 1367–1374 (2010).
Rauch, D. et al. Assessment of longitudinal bone growth in osteogenesis imperfecta using metacarpophalangeal pattern profiles. Bone 140, 115547 (2020).
Zeitlin, L., Rauch, F., Plotkin, H. & Glorieux, F. H. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics 111, 1030–1036 (2003).
Sanguinetti, C., Greco, F., De Palma, L., Specchia, N. & Falciglia, F. Morphological changes in growth-plate cartilage in osteogenesis imperfecta. J. Bone Joint Surg. Br. 72, 475–479 (1990).
Grafe, I. et al. Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 20, 670–675 (2014).
Tauer, J. T., Abdullah, S. & Rauch, F. Effect of anti-TGF-beta treatment in a mouse model of severe osteogenesis imperfecta. J. Bone Min. Res. 34, 207–214 (2019).
Wang, W., Rigueur, D. & Lyons, K. M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. C. Embryo Today 102, 37–51 (2014).
Nicol, L. E. et al. Alterations of a serum marker of collagen X in growing children with osteogenesis imperfecta. Bone 149, 115990 (2021).
Palomo, T., Glorieux, F. H., Schoenau, E. & Rauch, F. Body composition in children and adolescents with osteogenesis imperfecta. J. Pediatr. 169, 232–237 (2016).
Tauer, J. T., Robinson, M. E. & Rauch, F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR 3, e10174 (2019).
Acknowledgements
We thank Michaela Durigova for organizational support.
Funding
This study was supported by the Shriners of North America and the Fonds de recherche du Québec – Santé. Shriners of North America and Fonds de recherche du Québec – Santé had no role in the design and conduct of the study.
Author information
Authors and Affiliations
Contributions
M.-E.R. assisted with data analysis and interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. D.R. collected the study data, completed the data analysis, assisted with the interpretation of the data, and reviewed and revised the manuscript. F.H.G. contributed patient information and reviewed and revised the manuscript. F.R. conceptualized and designed the study, contributed patient information, assisted with data analysis and interpretation, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
M.-E.R. declares study grants to institution from Ascendis Biopharma and Ipsen Biopharmaceuticals and consultancy fees to institution from Ipsen Biopharmaceuticals and Ultragenyx. D.R. has no conflicts of interest to declare. F.H.G. declares consulting fees and research grants from Novartis, Amgen, Mereo Biopharma and Ultragenyx. F.R. reports study grants to institution from PreciThera Inc, Ultragenyx Inc and Catabasis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robinson, ME., Rauch, D., Glorieux, F.H. et al. Standardized growth charts for children with osteogenesis imperfecta. Pediatr Res 94, 1075–1082 (2023). https://doi.org/10.1038/s41390-023-02550-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02550-0
This article is cited by
-
Psychosocial Outcomes of Pain and Pain Management in Adults with Osteogenesis Imperfecta: A Qualitative Study
Journal of Clinical Psychology in Medical Settings (2024)
-
Early Life Management of Osteogenesis Imperfecta
Current Osteoporosis Reports (2023)