Abstract
Background
Chorioamnionitis is associated with preterm delivery and morbidities; its role in lung disease is controversial. The aim of this study is to assess the effect of chorioamnionitis on metabolite and lipid profiles of epithelial lining fluid in preterm newborns with respiratory distress syndrome (RDS).
Methods
The study involved 30 newborns with RDS, born from mothers with or without histological chorioamnionitis (HCA): HCA+, N = 10; HCA−, N = 20. Patients had a gestational age ≤30 weeks; the groups were matched for age and birth weights. Tracheal aspirates were collected within 24 h after birth and analyzed using liquid chromatography/mass spectrometry-based untargeted lipidomics.
Results
According to Mann–Whitney U tests, 570 metabolite features had statistically significantly higher or lower concentrations (p < 0.05) in tracheal aspirates of HCA+ compared to HCA−, and 241 metabolite features were putatively annotated and classified. The most relevant changes involved higher levels of glycerophospholipids (fold change 2.42–17.69) and sphingolipids, with lower concentration of all annotated sphingomyelins in HCA+ (fold change 0.01–0.50).
Conclusions
Untargeted lipidomics of tracheal aspirates suggested the production of lipid mediators in the context of an ongoing inflammatory status in HCA+ babies. However, the effect of chorioamnionitis on epithelial lining fluid composition deserves further investigations on a larger group of infants.
Impact
-
Our lipidomics investigation on tracheal aspirates of preterm newborns at birth suggested that exposure to maternal histological chorioamnionitis may cause changes in epithelial lining fluid composition.
-
This is the first description of epithelial lining fluid lipidomic profiles in preterm infants with and without exposition to chorioamnionitis.
-
These results could provide novel link between placental membrane inflammation and newborns’ respiratory outcome.
Similar content being viewed by others
Introduction
Chorioamnionitis is a complication of pregnancy characterized by neutrophil infiltration in the fetal membranes, with or without a fetal inflammatory response. It is characterized by the passage of organisms most commonly from the cervix or the vagina to the chorioamnion, and the fetal response will depend on both the type of organism and the duration of fetal exposure.1 It is a major risk factor for preterm delivery, with an incidence inversely related to gestational age (GA), and it has been associated with adverse perinatal outcome in preterm infants.2 The association with lung disease remains to be clarified. It has been reported that chorioamnionitis correlates with both a decreased risk of respiratory distress syndrome (RDS) and an increased risk of chronic lung disease in preterm infants.3,4 However, other studies reported no specific effects on the development of chronic lung diseases.5
RDS is a breathing disorder characterized by lung immaturity and deficiency of lung surfactant, the lipoprotein component of epithelial lining fluid (ELF). In animal models, experimentally induced chorioamnionitis with intra-amniotic application of endotoxin or interleukin-1 is associated with increased lung maturation in sheep6 and an increase in surfactant lipids7 and mRNA for surfactant proteins.8 This is in agreement with the decreased incidence of RDS and the lower early oxygen requirement found in preterm infants exposed to chorioamnionitis. On the other hand, the exposure to intrauterine infection seems to make the fetal lung prone to cytokine-mediated inflammation, along with a subsequent increased risk of chronic lung diseases.9
Lipidomics and metabolomics are good approaches to identify metabolic changes related to a particular pathophysiological state. A pilot study on metabolomics of urines from preterm newborns born from mothers with or without histological chorioamnionitis (HCA) reported a decrease in concentration for 29 metabolites related to mitochondrial electron transport chain, citric acid cycle, and glutamate and carbohydrate metabolism in newborns exposed to chorioamnionitis.10
When ethically feasible, the use of biospecimen closer to the target organ is usually preferable to proximal biofluids, such as plasma or urine, to investigate specific metabolic perturbations.11 Tracheal aspirates (TAs) are good proxies of ELF composition12 and could reflect in a more accurate way the effect of intrauterine inflammation on the newborn lungs. We recently reported that preterm newborns exposed to HCA had higher concentrations of ELF disaturated phosphatidylcholine (DSPC), surfactant protein B, and myeloperoxidase activity, suggesting a stimulated lung maturation in these babies.13 Moreover, exposure to chorioamnionitis was associated with an elevated presence of pro-inflammatory cells, cytokines, and prostaglandins in TAs.3,14 However, information on TA metabolomics are scarce.15,16
The aim of the present study was to assess the effect of chorioamnionitis on the lipid profiles of TAs in preterm newborns affected by RDS.
Methods
Supporting material with detailed methods is available as Supplementary Material (online).
Study population
The study protocol was approved by the Local Ethics Committees (Prot. no. 1591P and no. 382) and informed consent was obtained from parents. Prospective recruitment took place from 2014 to 2017 at the Neonatal Intensive Care Unit, Department of Women’s and Children’s Health, University Hospital of Padova and at the Neonatal Intensive Care, Salesi Children’s Hospital, Ancona, Italy. Selected case infants were preterm newborns with GA >23 and ≤30 weeks who needed intubation for RDS and met the following criteria at delivery: blood pH > 7.20, 5-min Apgar score ≥ 5, cesarean section delivery to minimize the risk of perinatal hypoxic–ischemic insult. Exclusion criteria were severe congenital malformations, chromosomal abnormalities, and lack of placenta histological examination report.
Based on the diagnosis of HCA stage 2 and 3, as reported in Supplementary Material (involving the chorionic plate and/or the umbilical cord), infants were divided into two groups: positive for HCA (HCA+) or negative for HCA (HCA−). Moreover, the two groups were matched for GA, birth weight, sex, clinical characteristics, drugs and resuscitation strategies during the perinatal period, and time of TA sampling, since we assumed that all these additional variables could have affected the results of an untargeted analysis. This stringent inclusion criteria led to a selection of a HCA+ group where nine out of ten mothers had also a clinical diagnosis of chorioamnionitis. Criteria for the diagnosis of RDS, clinical chorioamnionitis, and HCA are provided in online supporting material. The basic clinical characteristics are reported in Table 1.
Sample collection
For lipidomic and metabolomic investigations, TAs were collected within the first 24 h of life, immediately after intubation and before exogenous surfactant administration, as previously reported.17
For plasma to TA urea ratio analysis, the leftover blood from arterial blood gas analysis closest in time to the TA sample and within 4 h from the time of TA sample was collected.
Urea analysis
TA sampling collection procedure, by instillation of saline solution, causes an unknown dilution of ELF components. To circumvent this problem, we used the ratio of plasma to TA urea concentration as an estimation of sample dilution factor. Since urea is a plasma component that freely diffuses throughout the body, including the lung, the ratio between plasma and TA urea concentrations can be used as a dilution factor of recovered ELF.18
Plasma and TA urea was analyzed using a colorimetric assay (QuantiChrom TM Urea Assay Kit, BioAssay Systems, Hayward, CA).
Untargeted metabolomics analysis of TA samples
Sample preparation
Briefly, two different 40 µl aliquots of TA sample were deproteinized adding cold high-performance liquid chromatography (HPLC)-grade acetonitrile or 2-isopropanol for water-soluble metabolite and lipid profiling, respectively. An aliquot from each deproteinized sample was pooled to construct a pooled quality control (QC) sample.19 Process blank samples were prepared using the same solvent and procedure used for TA samples but with the absence of any biological sample.
Sample analysis
Ultra-HPLC–tandem mass spectrometry (UHPLC-MS(/MS)) analysis were performed on a Vanquish UHPLC system (Thermo Fisher Scientific, Bremen, Germany) coupled to an electrospray ionization (ESI) Q Exactive Plus mass spectrometer (Thermo Fisher scientific, Bremen, Germany) for water-soluble metabolite profiling (HILIC ESI+ and HILIC ESI−) and lipid profiling (LIPIDS ESI+ and LIPIDS ESI−).
Data processing and statistical analysis
Data processing was performed for each analytical batch (LIPIDS ESI+, LIPIDS ESI−, HILIC ESI+, and HILIC ESI−) separately. The filtered and normalized datasets were analyzed in MetaboAnalyst20 applying the unsupervised multivariate analysis technique principal components analysis (PCA). Volcano plots were constructed to assess statistically significant differences between the two groups (p value < 0.05 and fold change (FC) HCA+/HCA− <0.9 or >1.1). Volcano plot analysis was repeated applying correction for false discovery rate (FDR) using Benjamini–Hochberg procedure (adjusted p value threshold 0.05). Statistically significant metabolite features were annotated according to level 2 of the MSI reporting standards21 using the in-house software at the University of Birmingham (BEAMS).
Results
TA samples from 30 newborns affected by RDS and born from mothers with HCA (HCA+, N = 10) or without HCA (HCA−, N = 20) were analyzed via UHPLC/ESI-MS untargeted metabolomic and lipidomic approaches in both ESI+ and ESI−. The use of four complementary UHPLC/ESI-MS assays provided a broad coverage of water-soluble (HILIC ESI+ and HILIC ESI−) and lipid (LIPIDS ESI+ and LIPIDS ESI−) metabolites.
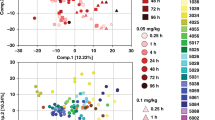
After data filtering using blank and pooled QC samples, we detected over 4000 unique features across all four datasets. The PCA analysis for each dataset (LIPIDS ESI+, LIPIDS ESI−, HILIC ESI+, HILIC ESI−) showed a tight clustering for pooled QC samples relative to the observed dispersion of biological samples, reflecting a system stability and reproducibility of sample preparation (see Fig. S1 in Supplementary Material). Small deviations from the origin of the PCA score plot can be due to sample weight discrepancies, since normalization for TA sample dilution factor was performed post-acquisition, during data processing. According to PCA, a sample from HCA+ group (HCA+_8) was observed to be an outlier for both lipid ESI+ and lipid ESI−, and it was removed from both lipid datasets prior to statistical analysis. Three samples (1 from the HCA+ group and 2 from the HCA− group) were removed from the HILIC ESI+ dataset, since the number of metabolite features detected was <20% of the average for all biological samples.
Taken together, the volcano plot analyses for the 4 datasets identified a total of 570 metabolite features present at a statistically significantly higher or lower concentration in the ELF of the HCA+ group compared to HCA−. Most of these metabolite features were detected by lipid profiling (334 and 125 for LIPIDS ESI+ and LIPIDS ESI−, respectively), with a lower contribution from water-soluble metabolite profiling (36 and 75 for HILIC ESI+ and HILIC ESI−, respectively). Of the 570 statistically different metabolic features, 241 were putatively annotated and classified (Fig. 1). The list of putatively annotated compounds present at lower or higher concentrations in HCA+ with obtained FC and p value is reported in Tables S1 and S2 available as online Supplementary Material, respectively. Of the 134 metabolites present at a statistically significantly higher concentration in the ELF of the HCA+ group, 58% was represented by glycerophospholipids (FC 2.42–17.69) and 9% by ceramides (FC 2.12–46.71). Among the 107 metabolites present at a statistically significantly lower concentration in the ELF of the HCA+ group, sphingolipids covered the highest percentage (30%), with 15 sphingomyelins (SMs; FC 0.01–0.50) and 16 ceramides (FC 0.07–0.52), 17% were glycerophospholipids (FC 0.10–0.73), 8% diacylglycerides (DGs, FC 0.14–0.71), 7% short-chain (chain length C2–C8) acyl carnitines (FC 0.29–0.53), and about 5% metabolites associated with ubiquinone or other terpenoid-quinone biosynthesis (FC 0.33–0.57).
After applying correction for FDR to the volcano plot analysis, only two unique features were statistically significant. One was putatively annotated as a SM (FC 0.01, adjusted p value 0.046), the second one was unidentified (FC 0.19, adjusted p value 0.046).
Discussion
Fetal exposure to maternal HCA can affect fetal development resulting in poor outcome for the newborn, such as the onset of neonatal sepsis or increased incidence of early neurologic insults. The association between HCA and lung diseases has been studied extensively, but the results remain inconsistent. The aim of this study was to explore changes in lipidomic and metabolomic profiles in preterm infants with and without HCA. We observed statistically significant changes in lipid and metabolic profiles between the two groups, although there were only two metabolites that were significantly different when a FDR correction was applied.
Most of the ELF changes were lipids. In the lungs, lipids play crucial roles in many processes with unique profiles across lung cell types.22 They represent the primary component of pulmonary surfactant (~90%), with glycerophospholipids being the most represented species (~80–85% by weight) and DSPC accounting for about 50% of total phospholipids (PLs). Of the DSPCs, dipalmitoyl-phosphatidylcholine (DPPC) is the most abundant species.23 We recently reported a higher DSPC concentration in the ELF of newborns exposed to HCA compared to age-matched infants born from HCA− mothers.13 In the present study, the difference in putatively annotated ELF DPPC ([M + H]+, m/z 734.5689, rt 415.09 s) did not reach the statistical significance. However, the median peak intensity [interquartile range (IQR)] was higher in HCA+ (4.7E + 11 [1.5E + 11 − 1.1E + 12]) than in HCA− (2.8E + 10 [1.6E + 10 − 1.9E + 11]), according to the hypothesis of a stimulated lung maturation mediated by the prenatal infection, although the difference was not statistically significant. Moreover, 78 different glycerophospholipids were at a higher concentration in the HCA+ group. Among these, 16 were phosphatidylserines (PSs). The exact physiological role of PSs in lung function and surfactant metabolism is not completely understood, although it has been reported that Ca2+-PS-dependent protein kinase may be involved in the initiation or regulation of synthesis of surfactant components, in particular phosphatidylcholine in fetal lung cells.24 Phosphatidylglycerol (PG) is the second most abundant lipid species in lung surfactant (up to 11% of lipids), primarily esterified by palmitate (C16:0) and oleate (C18:1ω9). PG enhances the spread of PL on the alveolar surface, but it is usually detectable only near term. At this stage, the higher presence of PG species having longer unsaturated chains, together with other acidic PLs, such as phosphatidylinositols (PIs), could be related to enhanced innate immune response in HCA+ babies. Indeed, surfactant anionic PLs, such as PG and PI, have been recently described as regulators of innate immune processes within the alveolar compartment, where they inhibit lipopolysaccharide (LPS)-induced cell activation by competitive binding to LPS receptors.25 Numata and colleagues26 have suggested that this inhibition prevents contrived recruitment and activation of neutrophils and other inflammatory cells, which release a mixture of proteases, cytokines, and reactive oxygen species that eliminate pathogens but, on the other hand, can cause damage to the alveolar epithelium. Complex glycerophospholipids containing long-chain polyunsaturated fatty acids have been reported in fetal mice lung27 and have been linked to inflammatory processes, acting as substrates for the generations of lipid mediators having both pro-inflammatory and pro-resolution functions.28
Sphingolipids represent a small portion (2–3%) of structural lipids of lung surfactant. However, their concentration may increase in the setting of acute or chronic lung injury and during lung development and they have been used as a marker for both pulmonary development and disease.29 In particular, SM levels increase during lung development in rats with the highest levels at birth, due to both an increase in its rate of biosynthesis and a decreased activity of enzymes involved in SM degradation.30 The bioactive sphingolipids also include ceramides, sphingosine, sphingosine-1-phosphate, ceramide-1-phosphate, and others. SM hydrolysis has been reported as a potentially important effector pathway for stimulatory factors associated with acute lung injury, such as tumor necrosis factor-α (TNF-α), Fas/Apo ligand, and ionizing radiation. Indeed, TNF-α activates a sphingomyelinase that hydrolyzes SM to ceramide; ceramide can in turn rapidly deacylate to sphingosine. Although sphingolipids are mainly generated intracellularly, Ryan and colleagues31 reported the presence of an alveolar secretory constitutively active sphingomyelinase and of a cell-free alveolar pool of sphingolipids regulated by TNF-α. Their results suggest that the activation of this sphingomyelinase could induce the production of alveolar ceramides that could play an important role in the early phase of cytokine-mediated acute lung injury. Interestingly, in the present study, all the annotated SMs were decreased in the HCA+ group, while long-chain ceramides were significantly increased and very-long-chain ceramides significantly decreased. Moreover, putatively annotated sphingosine d18:1 ([M + H]+, m/z 300.2898, rt 182.91 s) was detected in 67% of HCA+ patients versus 35% in the HCA− group, with higher median peak intensity [IQR] in HCA+ (2.4E + 07 [1.4E + 07 − 4.0E + 07]) than in HCA− (7.2E + 06 [4.5E + 06 − 1.5E + 07]), although the difference was not statistically significant. It is known that the exposure to intrauterine infection makes the fetal lung prone to cytokine-mediated inflammation. Thus, from this preliminary work, it is conceivable to hypothesize a tendency to an enhanced production of lipid mediators in the context of an ongoing inflammatory status in HCA+ babies.
Additionally, among annotated ceramides, galabiosylceramides/lactosylceramides (LacCer) showed the strongest significant FC (up to 46.7) in the ELF of the HCA+ group. This is in agreement with data obtained in amniotic fluid metabolomics of HCA+ women, where LacCer(d18:1/16:0) and LacCer(d18:1/24:1) were suggested as biomarker candidates for predicting subclinical chorioamnionitis.32
Among the other classes of metabolites, carnitine and short-chain acylcarnitines (C2–C8) were lower in the ELF of HCA+ newborns. Carnitines are present in cells and tissues as both free carnitine and acylcarnitines, and its most widely known function is the transport of long-chain fatty acids into mitochondria for β-oxidation. However, other possible roles have been reported. Short-chain acylcarnitines have been previously described in both lung and bronchoalveolar lavages in a murine model of influenza pneumonia, indicating a possible role in the host response to the infection.33 In some tissues, short-chain acylcarnitines may be used for energy and as a carbon source for PL synthesis.34 While medium- and long-chain acylcarnitines are derived exclusively from fatty acid metabolism, short-chain acylcarnitines are produced from glucose, amino acid, and fatty acid degradation. Under physiological conditions, an organism switches between glucose and fatty acid metabolism based on the availability of substrates to maintain energy homeostasis.35 Differences in acylcarnitines levels might be related to specific changes in energy metabolism. Another possibility is that the presence of short-chain acylcarnitines in TAs may be related to the presence of bacteria. That would also explain the presence of annotated complex lipids with odd chain fatty acids, usually uncommon in human tissues but attributable either to bacterial contamination during the sampling procedure36 or to the airway microbiome. The study of the human lung microbiome is an area of emerging research. It has been reported that fetus swallows large amounts of amniotic fluid leading to the colonization of the gut with intrauterine microbiome already before birth. Its microbiome is then highly influenced by the microbiome of the amniotic fluid. Especially in case of maternal infection, the fetus is exposed to an increased bacteria abundance and experiences an immunologic adaptation. Previous studies showed increased abundance of bacteria but decreased diversity in airway microbiome of infants exposed to chorioamnionitis and reduced microbial diversity at birth in TAs of preterm infants, especially in association with higher severity of bronchopulmonary dysplasia.37,38 Thus the presence of short-chain acylcarnitines and lipids of odd chain fatty acids in our samples can be related to the lung microbiome.
This study has some limitations. First, the small number of patients included in the study could have affected the statistical analysis. In clinical practice, obtaining samples from unbiased matched patients extremely reduces the number of available cases, especially when such a sensitive population, as preterm infants, and specific biospecimen are involved. Nevertheless, the major strength of the present study is that the two groups were accurately matched, in terms of clinical characteristics, drugs and resuscitation strategies during the perinatal period, and time of TA sampling. More importantly, the diagnosis of chorioamnionitis included both clinical and histological criteria. Intubation, oxygen supplementation, and asphyxia at birth are pro-inflammatory factors that trigger inflammatory pathways, making it difficult to discriminate between secondary inflammation at birth and the effects of chorioamnionitis. As shown in Table 1, all newborns had a cesarean section and the two study groups were almost identical in terms of oxygen supplementation, need of mechanical ventilation in the delivery room, and Apgar scores; therefore, it is conceivable that the changes in ELF lipid profiles were related to the presence of the chorioamnionitis. Moreover, this is the first description of ELF lipidomic profiles in preterm infants with and without exposition to chorioamnionitis in an unbiased and accurately defined cohort of preterm infants.
Another limitation is that a post-acquisition normalization method was applied to correct for sample dilution. However, post analysis normalization has been extensively used for urine metabolomics, and TA/plasma urea concentration has been reported to be a reliable marker of dilution.18
Because of these limitations, the present study has to be considered as an explorative study on the application of untargeted lipidomics on TA samples. This approach could be promising to identify pathways that can be altered in newborns because of maternal infection. Although we found changes in specific areas of lipid metabolism, the hypothesis of an effect of chorioamnionitis on ELF lipidomics/metabolomics deserves a further unbiased investigation on a larger group of patients.
References
Jobe, A. Effects of chorioamnionitis on the fetal lung. Clin. Perinatol. 39, 441–457 (2012).
Dempsey, E. et al. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am. J. Perinatol. 22, 155–159 (2005).
Watterberg, K. L., Demers, L. M., Scott, S. M. & Murphy, S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97, 210–215 (1996).
Lahra, M. M., Beeby, P. J., Jeffery, H. E. & Lahra, M. M. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study. Arch. Dis. Child. Fetal Neonatal 94, F13–F16 (2009).
Kent, A. & Dahlstrom, J. Chorioamnionitis/funisitis and the development of bronchopulmonary dysplasia. J. Paediatr. Child Health 40, 356–359 (2004).
Kramer, B. Antenatal inflammation and lung injury: prenatal origin of neonatal disease. J. Perinatol. 28, S21–S27 (2008).
Jobe, A. H. et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am. J. Respir. Crit. Care Med. 162, 1656–1661 (2000).
Bachurski, C. J., Ross, G. F., Ikegami, M., Kramer, B. W. & Jobe, A. H. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L279–L285 (2001).
Speer, C. P. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin. Fetal Neonatal Med. 11, 354–362 (2006).
Fattuoni, C. et al. Urinary metabolomic analysis to identify preterm neonates exposed to histological chorioamnionitis: a pilot study. PLoS ONE 12, e0189120 (2017).
Chetwynd, A. J., Dunn, W. B. & Rodriguez-Blanco, G. In Metabolomics: From Fundamentals to Clinical Applications. Advances in Experimental Medicine and Biology (ed. Sussulini, A.) 19–44 (Springer, 2017).
Dargaville, P. A., South, M. & McDougall, P. N. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am. J. Respir. Crit. Care Med. 160, 771–777 (1999).
Verlato, G. et al. Surfactant components and tracheal aspirate inflammatory markers in preterm infants with respiratory distress syndrome. J. Pediatr. 203, 442–446 (2018).
De Dooy, J. et al. Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr. Res. 54, 113–119 (2003).
Lal, C. V. et al. Early airway microbial metagenomic and metabolomic signatures are associated with development of severe bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, 810–815 (2018).
Piersigilli, F. et al. Identification of new biomarkers of bronchopulmonary dysplasia using metabolomics. Metabolomics 15, 0 (2019).
Simonato, M. et al. Influence of the type of congenital heart defects on epithelial lining fluid composition in infants undergoing cardiac surgery with cardiopulmonary bypass. Pediatr. Res. 83, 791–797 (2018).
Dargaville, P. A., South, M., Vervaart, P. & Dougall, P. N. M. C. Validity of markers of dilution in small volume lung lavage. Am. J. Respir. Crit. Care Med. 160, 778–784 (1999).
Broadhurst, D. et al. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 14, 72 (2018).
Chong, J., Wishart, D. S. & Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinformatics 68, e86 (2019).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) NIH Public Access. Metabolomics 3, 211–221 (2007).
Kyle, J. E. et al. Cell type-resolved human lung lipidome reveals cellular cooperation in lung function. Sci. Rep. 8, 13455 (2018).
Vedovelli, L. et al. Simultaneous measurement of phosphatidylglycerol and disaturated-phosphatidylcholine palmitate kinetics from alveolar surfactant. Study in infants with stable isotope tracer, coupled with isotope ratio mass spectrometry. J. Mass Spectrom. 46, 986–992 (2011).
Samuels, E. R. & Elliott Scott, J. Ca+2-phosphatidylserine-dependent protein kinase C activfty in fetal, neonatal and adult rabbit lung and isolated lamellar bodies. Life Sci. 57, 1557–1568 (1995).
Kuronuma, K. et al. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 284, 25488–25500 (2009).
Numata, M., Kandasamy, P. & Voelker, D. R. Anionic pulmonary surfactant lipid regulation of innate immunity. Expert Rev. Respir. Med. 6, 243–246 (2012).
Dautel, S. E. et al. Lipidomics reveals dramatic lipid compositional changes in the maturing postnatal lung. Sci. Rep. 7, 1–12 (2017).
Serhan, C. N., Chiang, N. & Van Dyke, T. E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 (2008).
Uhlig, S. & Gulbins, E. Sphingolipids in the lungs. Am. J. Respir. Crit. Care Med. 178, 1100–1114 (2008).
Longo, C. A., Tyler, D. & Mallampalli, R. K. Sphingomyelin metabolism is developmentally regulated in rat lung. Am. J. Respir. Cell Mol. Biol. 16, 605–612 (1997).
Ryan, A. J., McCoy, D. M., McGowan, S. E., Salome, R. G. & Mallampalli, R. K. Alveolar sphingolipids generated in response to TNF-α modifies surfactant biophysical activity. J. Appl. Physiol. 94, 253–258 (2003).
Dudzik, D., Revello, R., Barbas, C. & Bartha, J. L. LC−MS-based metabolomics identification of novel biomarkers of chorioamnionitis and its associated perinatal neurological damage. J. Proteome Res. 14, 1432–1444 (2015).
Cui, L. et al. Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci. Rep. 6, 1–13 (2016).
Jones, L. L., McDonald, D. A. & Borum, P. R. Acylcarnitines: role in brain. Prog. Lipid Res. 49, 61–75 (2010).
Makrecka-Kuka, M. et al. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep. 7, 17528 (2017).
Brandsma, J. et al. Lipid phenotyping of lung epithelial lining fluid in healthy human volunteers. Metabolomics 14, 123 (2018).
Lal, C. V. et al. The airway microbiome at birth. Sci. Rep. 6, 1–13 (2016).
Staude, B. et al. The microbiome and preterm birth: a change in paradigm with profound implications for pathophysiologic concepts and novel therapeutic strategies. Biomed. Res. Int. 2018, 1–12 (2018).
Acknowledgements
The study was performed with no specific financial support.
Author information
Authors and Affiliations
Contributions
Conception and design: V.P.C., P.C., W.B.D. Patient recruitment and data collection: G.V., L.B. Laboratory experiments: S.G., L.N., L.V. Analysis and interpretation: S.G., W.B.D., P.C., M.S. Drafting of the manuscript: S.G., P.C. Revision of the manuscript and final approval: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed consent was obtained from parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Giambelluca, S., Verlato, G., Simonato, M. et al. Chorioamnionitis alters lung surfactant lipidome in newborns with respiratory distress syndrome. Pediatr Res 90, 1039–1043 (2021). https://doi.org/10.1038/s41390-021-01371-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01371-3
This article is cited by
-
Omics approaches: interactions at the maternal–fetal interface and origins of child health and disease
Pediatric Research (2023)
-
Chorioamnionitis and neonatal outcomes
Pediatric Research (2022)