Abstract
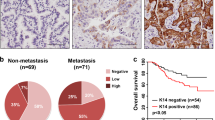
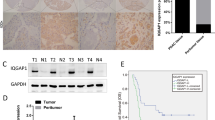
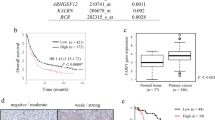
DGC is a particular aggressive malignancy with poor prognosis. Recent omics studies characterized DGC with CDH1/E-cadherin loss and EMT-signatures. However, the underlying mechanisms for maintaining the aggressive behavior and molecular features of DGC remain unclear. Here, we find that intermediate filaments KRT17 is significantly lower in DGC tissues than that in intestinal gastric cancer tissues and associated with poor prognosis of DGC. We demonstrate that downregulation of KRT17 induces E-cadherin loss, EMT changes, and metastasis behaviors of GC cells. Mechanistically, the loss of intermediate filaments KRT17 induces reorganization of cytoskeleton, further activates YAP signaling, and increases IL6 expression, which contributes to the enhanced metastasis ability of GC cells. Together, these results indicate that KRT17/YAP/IL6 axis contributes to maintaining E-cadherin loss, EMT feature, and metastasis of DGC, providing a new insight into the role of aberrant intermediate filaments in DGC malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The accession number of RNA-sequencing raw data in this study is No. GSE171879.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. APMIS. 1965;64:31–49.
Henson DE, Dittus C, Younes M, Nguyen H, Alboressaavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2009;128:765–70.
Lee JY, Gong EJ, Chung EJ, Park HW, Bae SE, Kim EH, et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-ups. Gut Liver. 2017;11:807–12.
Cristescu R, Lee J, Nebozhyn M, Kim K, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56.
Bass AJ, Thorsson V, Shmulevich I, Reynolds S, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9.
Mun DG, Bhin J, Kim S, Kim H, Jung JH, Jung Y, et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell. 2019;35:111.
Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng J, et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9:1012.
Harvey KF, Zhang X, Thomas DM. The hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Johnson RL, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79.
Park J, Kim DH, Shah SR, Kim HN, Kshitiz, Kim P, et al. Switch-like enhancement of epithelial-mesenchymal transition by YAP through feedback regulation of WT1 and Rho-family GTPases. Nat Commun. 2019; 10: 2797.
Yang N, Chen T, Wang L, Liu R, Niu Y, Sun L. et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10:5790–5801.
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The hippo transducer taz confers cancer stem cell-related traits on breast cancer cells. Cell 2011;147:759–72.
Qi YJ, Jiao YL, Chen P, Kong JY, Gu BL, Liu K. et al. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFá-dependent Smad/YAP/TAZ signaling. PLoS Biol. 2020;18:e3000825.
Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepemooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell 2014;157:1324–38.
Zhao B, Li L, Wang LH, Wang C, Yu J, Guan K. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68.
Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a yap antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80.
Kang W, Tong JHM, Chan AWH, Lee T-L, Lung RWM, Leung PPS, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130–9.
Zhang H, Schaefer A, Wang Y, Hodge RG, Blake DR, Diehl JN. et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 2020;10:288–305.
Yang T, Gemin F, Fenghua G, Hui Z, Xiaoxu C, Liwei A. et al. Selective inhibition of strn3-containing pp2a phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell. 2020;38:115–128.e9.
Choi W, Kim J, Park J, Lee D-H, Hwang D, Kim J-H. et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Res. 2018;78:3306–20.
Huang C, Yuan W, Lai C, Zhong S, Yang C, Wang R, et al. Epha2-to-yap pathway drives gastric cancer growth and therapy resistance. Int J Cancer. 2020;146:1937–49.
Jiao S, Guan J, Chen M, Wang W, Li C, Wang Y, et al. Targeting IRF3 as a yap agonist therapy against gastric cancer. J Exp Med. 2018;215:699–718.
Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in drosophila via the ferm-domain protein expanded. Curr Biol. 2010;20:582–90.
Mohseni M, Sun J, Lau AN, Curtis SJ, Goldsmith JD, Fox VL, et al. A genetic screen identifies an LKB1–MARK signalling axis controlling the Hippo–YAP pathway. Nat Cell Biol. 2014;16:108–17.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of yap oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Yue T, Tian A, Jiang J. The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the hippo signaling pathway. Dev Cell. 2012;22:255–67.
Yu F-X, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the hippo-yap pathway by g-protein-coupled receptor signaling. Cell 2012;150:780–91.
Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Investig. 2015;125:2123–35.
Park HW, Kim Y, Yu B, Moroishi T, Mo J, Plouffe SW, et al. Alternative WNT signaling activates YAP/TAZ. Cell 2015;162:780–94.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013;154:1047–59.
Kim HJ, Choi WJ, Lee CH. Phosphorylation and reorganization of keratin networks: Implications for carcinogenesis and epithelial mesenchymal transition. Biomol Ther. 2015;23:301–12.
Kurokawa I, Takahashi K, Moll I, Moll R. Expression of keratins in cutaneous epithelial tumors and related disorders–distribution and clinical significance. Exp Dermatol. 2011;20:217–28.
Proby CM, Churchill LJ, Purkis PE, Glover MT, Sexton CJ, Leigh IM. Keratin 17 expression as a marker for epithelial transformation in viral warts. Am J Pathol. 1993;143:1667–78.
Hobbs RP, Batazzi AS, Han MC, Coulombe PA. Loss of keratin 17 induces tissue-specific cytokine polarization and cellular differentiation in hpv16-driven cervical tumorigenesis in vivo. Oncogene 2016;35:5653–62.
Chivueconomescu M, Dragu DL, Necula LG, Matei L, Enciu A, Bleotu C, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20:948–59.
Hu H, Xu D, Huang X, Zhu C, Xu J, Zhang Z, et al. Keratin17 promotes tumor growth and is associated with poor prognosis in gastric cancer. J Cancer. 2018;9:346–57.
Ide M, Kato T, Ogata K, Mochiki E, Kuwano H, Oyama T. Keratin 17 expression correlates with tumor progression and poor prognosis in gastric adenocarcinoma. Ann Surg Oncol. 2012;19:3506–14.
Chivu Economescu M, Necula LG, Dragu D, Badea L, Dima SO, Tudor S, et al. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453–64.
Khanom R, Nguyen CTK, Kayamori K, Zhao X, Morita K, Miki Y, et al. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLOS ONE. 2016; 11.
Wang Z, Yang M, Lei L, Fei L, Zheng Y, Huang W, et al. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Manag Res. 2019;11:7485–97.
Li D, Ni X, Tang H, Zhang J, Zheng C, Lin J, et al. KRT17 functions as a tumor promoter and regulates proliferation, migration and invasion in pancreatic cancer via mTOR/S6k1 pathway. Cancer Manag Res. 2020;12:2087–95.
Liu Z, Yu S, Ye S, Shen Z, Gao L, Han Z, et al. Keratin 17 activates AKT signalling and induces epithelial-mesenchymal transition in oesophageal squamous cell carcinoma. J Proteom. 2020;211:103557.
Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–52.
Cho SY, Park JW, Liu Y, Park YS, Kim JH, Yang H, et al. Sporadic early-onset diffuse gastric cancers have high frequency of somatic CDH1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology 2017;153:536.
Shimada S, Mimata A, Sekine M, Mogushi K, Akiyama Y, Fukamachi H, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut 2012;61:344–53.
Xu X, Qian L, Su X, He K, Jin K, Gu L, et al. Establishment and characterization of gcsr1, a multi-drug resistant signet ring cell gastric cancer cell line. Int J Oncol. 2015;46:2479–87.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011;27:1739–40.
Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature 2003;422:766–74.
Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–42.
Yu F, Zhao B, Guan K. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28.
Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914–21.
Wang Y, Chen D, Zhang Y, Wang P, Zheng C, Zhang S, et al. Novel adipokine, FAM19A5, inhibits neointima formation after injury through sphingosine-1-phosphate receptor 2. Circulation 2018;138:48–63.
Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, et al. Il-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol. 2016;11:1482–92.
Kim T, Yang SJ, Hwang D, Song J, Kim M, Kim SK, et al. A basal-like breast cancer-specific role for SRF–il6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186–10186.
Weng Y, Tseng H, Chen Y, Shen P, Haq ATA, Chen L, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. 2019;18:42.
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–94.
Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg. 2018;267:105–13.
Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–44.
Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–e70.
Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, et al. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868–75.
Lee KH, Hwang D, Kang KY, Lee S, Kim DY, Joo YE, et al. Frequent promoter methylation of cdh1 in non-neoplastic mucosa of sporadic diffuse gastric cancer. Anticancer Res. 2013;33:3765–74.
Quinn JJ, Jones MG, Okimoto RA, Nanjo S, Chan MM, Yosef N, et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science. 2021; 371.
van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline cdh1 mutation carriers. J Med Genet. 2015;52:361–74.
Mahmud N, Ford JM, Longacre TA, Parent R, Norton JA. Metastatic lobular breast carcinoma mimicking primary signet ring adenocarcinoma in a patient with a suspected CDH1 mutation. J Clin Oncol. 2015;33:e19–e21.
Lin M-T, Zuon C-Y, Chang C-C, Chen S-T, Chen C-P, Lin B-R, et al. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappaB/cyclooxygenase-2 signaling pathway. Clin Cancer Res. 2005;11:5809–20.
Yoon C, Cho S, Aksoy BA, Park DJ, Schultz N, Ryeom S, et al. Chemotherapy resistance in diffuse type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin Cancer Res. 2016;22:971–83.
Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces e-cadherin-dependent yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348:1024–7.
Mo J-S, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–10.
Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–70.
Fan R, Kim N-G, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110:2569–74.
Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–71.
Lim HYG, Alvarez YD, Gasnier M, Wang Y, Tetlak P, Bissiere S, et al. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 2020;585:404–9.
Eliazer S, Muncie JM, Christensen J, Sun X, D’Urso RS, Weaver VM, et al. Wnt4 from the niche controls the mechano-properties and quiescent state of muscle stem cells. Cell Stem Cell. 2019; 25.
Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–66.
Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X. et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111:E89–E98.
Wang K, Yuen ST, Xu J, Lee SP, Yan HHN, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82.
Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain-of-function mutations of RhoA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–7.
Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM, Choi KS, et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019;18:68.
Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–3.
Acknowledgements
We thank Hai Song for providing pcDNA3.1-YAP plasmid. This work was supported by the National Natural Science Foundation of China (31771540, 81972276, 82173040, 91740205, 31620103911), Natural Scientific Foundation of Zhejiang Province, China (LYY19H310011), Fundamental Research Funds for the Central Universities (2021QNA7004).
Author information
Authors and Affiliations
Contributions
WZ, TZ, ML and XR designed the experiments and interpreted the results. ML, XR, YC, XL, YZ performed the experiments. ML, LZ, and XR performed the animal experiments. ML, XR, BW, LT analyzed the clinical data. TZ improved the project design. ML, WZ wrote the manuscript, which was further refined by all authors. WZ supervised the overall study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Rao, X., Cui, Y. et al. The keratin 17/YAP/IL6 axis contributes to E-cadherin loss and aggressiveness of diffuse gastric cancer. Oncogene 41, 770–781 (2022). https://doi.org/10.1038/s41388-021-02119-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02119-3