Abstract
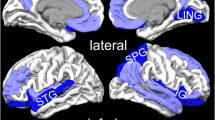
Posttraumatic stress disorder (PTSD) is associated with lower cortical thickness (CT) in prefrontal, cingulate, and insular cortices in diverse trauma-affected samples. However, some studies have failed to detect differences between PTSD patients and healthy controls or reported that PTSD is associated with greater CT. Using data-driven dimensionality reduction, we sought to conduct a well-powered study to identify vulnerable networks without regard to neuroanatomic boundaries. Moreover, this approach enabled us to avoid the excessive burden of multiple comparison correction that plagues vertex-wise methods. We derived structural covariance networks (SCNs) by applying non-negative matrix factorization (NMF) to CT data from 961 PTSD patients and 1124 trauma-exposed controls without PTSD. We used regression analyses to investigate associations between CT within SCNs and PTSD diagnosis (with and without accounting for the potential confounding effect of trauma type) and symptom severity in the full sample. We performed additional regression analyses in subsets of the data to examine associations between SCNs and comorbid depression, childhood trauma severity, and alcohol abuse. NMF identified 20 unbiased SCNs, which aligned closely with functionally defined brain networks. PTSD diagnosis was most strongly associated with diminished CT in SCNs that encompassed the bilateral superior frontal cortex, motor cortex, insular cortex, orbitofrontal cortex, medial occipital cortex, anterior cingulate cortex, and posterior cingulate cortex. CT in these networks was significantly negatively correlated with PTSD symptom severity. Collectively, these findings suggest that PTSD diagnosis is associated with widespread reductions in CT, particularly within prefrontal regulatory regions and broader emotion and sensory processing cortical regions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria. J Trauma Stress. 2013;26:537–47.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164:1476–88.
Karl A, Schaefer M, Malta L, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31.
O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2015;232:1–33.
Corbo V, Salat DH, Amick MM, Leritz EC, Milberg WP, McGlinchey RE. Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Res Neuroimaging. 2014;223:53–60.
Geuze E, Westenberg HGM, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–81.
Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, et al. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J Child Psychol Psychiatry. 2016;57:1154–64.
Sadeh N, Spielberg JM, Logue MW, Wolf EJ, Smith AK, Lusk J, et al. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Mol Psychiatry. 2016;21:357–63.
Sullivan DR, Morrison FG, Wolf EJ, Logue MW, Fortier CB, Salat DH, et al. The PPM1F gene moderates the association between PTSD and cortical thickness. J Affect Disord. 2019;259:201–9.
Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27:515–25.
Bing X, Ming-guo Q, Ye Z, Jing-na Z, Min L, Han C, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013;1490:225–32.
Crombie KM, Ross MC, Letkiewicz AM, Sartin-Tarm A, Cisler JM. Differential relationships of PTSD symptom clusters with cortical thickness and grey matter volumes among women with PTSD. Sci Rep. 2021;11:1825.
Ross MC, Sartin-Tarm AS, Letkiewicz AM, Crombie KM, Cisler JM. Distinct cortical thickness correlates of early life trauma exposure and posttraumatic stress disorder are shared among adolescent and adult females with interpersonal violence exposure. Neuropsychopharmacology. 2021;46:741–9.
Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601–11.
Clausen AN, Clarke E, Phillips RD, Haswell C, Morey RA. Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military Veterans. Neuropsychopharmacology. 2020;45:491–8.
Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller Global and Regional Cortical Volume in Combat-Related Posttraumatic Stress Disorder. Arch Gen Psychiatry. 2009;66:1373.
Dickie EW, Brunet A, Akerib V, Armony JL. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol Med. 2013;43:645–53.
Heyn SA, Herringa RJ. Longitudinal cortical markers of persistence and remission of pediatric PTSD. Neuroimage Clin. 2019;24:102028.
Jeong H, Lee YJ, Kim N, Jeon S, Jun JY, Yoo SY, et al. Increased medial prefrontal cortical thickness and resilience to traumatic experiences in North Korean refugees. Sci Rep. 2021;11:14910.
Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The Neurobiological Role of the Dorsolateral Prefrontal Cortex in Recovery From Trauma. Arch Gen Psychiatry. 2011;68:701.
Averill LA, Abdallah CG, Pietrzak RH, Averill CL, Southwick SM, Krystal JH, et al. Combat exposure severity is associated with reduced cortical thickness in combat veterans: a preliminary report. Chronic Stress. 2017;1:1–9.
Clouston SAP, Deri Y, Horton M, Tang C, Diminich E, DeLorenzo C, et al. Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimer’s & Dementia: Diagnosis. Assess Dis Monit. 2020;12:e12059.
Demers LA, Olson EA, Crowley DJ, Rauch SL, Rosso IM. Dorsal Anterior Cingulate Thickness Is Related to Alexithymia in Childhood Trauma-Related PTSD. PLoS One. 2015;10:e0139807.
Knight LK, Naaz F, Stoica T, Depue BE. Lifetime PTSD and geriatric depression symptomatology relate to altered dorsomedial frontal and amygdala morphometry. Psychiatry Res Neuroimaging. 2017;267:59–68.
Landré L, Destrieux C, Baudry M, Barantin L, Cottier J-P, Martineau J, et al. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Res Neuroimaging. 2010;183:181–6.
Rinne-Albers MA, Boateng CP, van der Werff SJ, Lamers-Winkelman F, Rombouts SA, Vermeiren RR, et al. Preserved cortical thickness, surface area and volume in adolescents with PTSD after childhood sexual abuse. Sci Rep. 2020;10:3266.
Rosada C, Bauer M, Golde S, Metz S, Roepke S, Otte C, et al. Childhood trauma and cortical thickness in healthy women, women with post-traumatic stress disorder, and women with borderline personality disorder. Psychoneuroendocrinology. 2023;153:106118.
Sun D, Haswell CC, Morey RA, De Bellis MD. Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Dev Psychopathol. 2019;31:557–71.
Li S, Huang X, Li L, Du F, Li J, Bi F, et al. Posttraumatic Stress Disorder: Structural Characterization with 3-T MR Imaging. Radiology. 2016;280:537–44.
Qi S, Mu Y, Liu K, Zhang J, Huan Y, Tan Q, et al. Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. Neuroimage Clin. 2013;3:226–33.
Li L, Zhang Y, Zhao Y, Li Z, Kemp GJ, Wu M, et al. Cortical thickness abnormalities in patients with post-traumatic stress disorder: A vertex-based meta-analysis. Neurosci Biobehav Rev. 2022;134:104519.
Galatzer-Levy IR, Bryant RA. 636,120 Ways to Have Posttraumatic Stress Disorder. Perspect Psychol Sci. 2013;8:651–62.
Sotiras A, Resnick SM, Davatzikos C. Finding imaging patterns of structural covariance via Non-Negative Matrix Factorization. Neuroimage. 2015;108:1–16.
Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci. 2017;114:3527–32.
Cui Z, Li H, Xia CH, Larsen B, Adebimpe A, Baum GL, et al. Individual Variation in Functional Topography of Association Networks in Youth. Neuron. 2020;106:340–53.e8.
Sun D, Adduru VR, Phillips RD, Bouchard HC, Sotiras A, Michael AM, et al. Adolescent alcohol use is linked to disruptions in age-appropriate cortical thinning: an unsupervised machine learning approach. Neuropsychopharmacology. 2023;48:317–26.
Kalantar-Hormozi H, Patel R, Dai A, Ziolkowski J, Dong H-M, Holmes A, et al. A cross-sectional and longitudinal study of human brain development: The integration of cortical thickness, surface area, gyrification index, and cortical curvature into a unified analytical framework. Neuroimage. 2023;268:119885.
Wang F, Lian C, Wu Z, Zhang H, Li T, Meng Y, et al. Developmental topography of cortical thickness during infancy. Proc Natl Acad Sci. 2019;116:15855–60.
Pehlivanova M, Wolf DH, Sotiras A, Kaczkurkin A, Moore TM, Ciric R, et al. Diminished Cortical Thickness is Associated with Impulsive Choice in Adolescence. J Neurosci. 2018;38:2200–17.
Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for Dissociable Linkage of Dimensions of Psychopathology to Brain Structure in Youths. Am J Psychiatry. 2019;176:1000–9.
Jirsaraie RJ, Kaczkurkin AN, Rush S, Piiwia K, Adebimpe A, Bassett DS, et al. Accelerated cortical thinning within structural brain networks is associated with irritability in youth. Neuropsychopharmacology. 2019;44:2254–62.
Luking KR, Jirsaraie RJ, Tillman R, Luby JL, Barch DM, Sotiras A. Timing and Type of Early Psychopathology Symptoms Predict Longitudinal Change in Cortical Thickness From Middle Childhood Into Early Adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:397–405.
Neufeld NH, Kaczkurkin AN, Sotiras A, Mulsant BH, Dickie EW, Flint AJ, et al. Structural brain networks in remitted psychotic depression. Neuropsychopharmacology. 2020;45:1223–31.
Yang W, Jin S, Duan W, Yu H, Ping L, Shen Z, et al. The effects of childhood maltreatment on cortical thickness and gray matter volume: a coordinate-based meta-analysis. Psychol Med. 2023;53:1681–99.
Li Q, Zhao Y, Chen Z, Long J, Dai J, Huang X, et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology. 2020;45:703–12.
Suh JS, Schneider MA, Minuzzi L, MacQueen GM, Strother SC, Kennedy SH, et al. Cortical thickness in major depressive disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:287–302.
Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1401–25.
Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The Co-Occurrence of Major Depressive Disorder Among Individuals With Posttraumatic Stress Disorder: A Meta-Analysis. J Trauma Stress. 2013;26:299–309.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244–53.
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Radua J, Vieta E, Shinohara R, Kochunov P, Quidé Y, Green MJ, et al. Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. Neuroimage. 2020;218:116956.
Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6.
Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/p.adjust.
Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113:7900–5.
Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Wang X, Xie H, Chen T, Cotton AS, Salminen LE, Logue MW, et al. Cortical volume abnormalities in posttraumatic stress disorder: an ENIGMA-psychiatric genomics consortium PTSD workgroup mega-analysis. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-00967-1.
Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. The genetic architecture of the human cerebral cortex. Science. 1979;2020:367.
Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2013;211:93–103.
Liberzon I, Abelson JL. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron. 2016;92:14–30.
Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–71.
Hooker CI, Knight RT. The role of lateral orbitofrontal cortex in the inhibitory control of emotion. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006. p. 307–24.
Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cogn Affect Behav Neurosci. 2002;2:264–70.
Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res Neuroimaging. 2012;203:139–45.
Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for Acquired Pregenual Anterior Cingulate Gray Matter Loss from a Twin Study of Combat-Related Posttraumatic Stress Disorder. Biol Psychiatry. 2008;63:550–6.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87.
Berman Z, Assaf Y, Tarrasch R, Joel D. Assault-related self-blame and its association with PTSD in sexually assaulted women: an MRI inquiry. Soc Cogn Affect Neurosci. 2018;13:775–84.
Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychol Rev. 2010;117:210–32.
Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2008;42:268–77.
Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, et al. Exaggerated Activation of Dorsal Anterior Cingulate Cortex During Cognitive Interference: A Monozygotic Twin Study of Posttraumatic Stress Disorder. Am J Psychiatry. 2011;168:979–85.
Harricharan S, Rabellino D, Frewen PA, Densmore M, Théberge J, McKinnon MC, et al. <scp>fMRI</scp> functional connectivity of the periaqueductal gray in <scp>PTSD</scp> and its dissociative subtype. Brain Behav. 2016;6:e00579.
Webb EK, Huggins AA, Belleau EL, Taubitz LE, Hanson JL, DeRoon-Cassini TA, et al. Acute Posttrauma Resting-State Functional Connectivity of Periaqueductal Gray Prospectively Predicts Posttraumatic Stress Disorder Symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:891–900.
Morey RA, Lancaster SC, Haswell CC. Trauma Re-experiencing Symptoms Modulate Topology of Intrinsic Functional Networks. Biol Psychiatry. 2015;78:156–8.
Todd RM, MacDonald MJ, Sedge P, Robertson A, Jetly R, Taylor MJ, et al. Soldiers With Posttraumatic Stress Disorder See a World Full of Threat: Magnetoencephalography Reveals Enhanced Tuning to Combat-Related Cues. Biol Psychiatry. 2015;78:821–9.
Herz N, Bar-Haim Y, Tavor I, Tik N, Sharon H, Holmes EA, et al. Neuromodulation of Visual Cortex Reduces the Intensity of Intrusive Memories. Cereb Cortex. 2022;32:408–17.
Badura-Brack A, McDermott TJ, Heinrichs-Graham E, Ryan TJ, Khanna MM, Pine DS, et al. Veterans with PTSD demonstrate amygdala hyperactivity while viewing threatening faces: A MEG study. Biol Psychol. 2018;132:228–32.
Lazarov A, Bar-Haim Y. Emerging Domain-Based Treatments for Pediatric Anxiety Disorders. Biol Psychiatry. 2021;89:716–25.
Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–95.
Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage. 2011;57:1492–8.
Weisberg SM, Ekstrom AD. Hippocampal volume and navigational ability: The map(ping) is not to scale. Neurosci Biobehav Rev. 2021;126:102–12.
Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48.
Elhai JD, de Francisco Carvalho L, Miguel FK, Palmieri PA, Primi R, Frueh BC. Testing whether posttraumatic stress disorder and major depressive disorder are similar or unique constructs. J Anxiety Disord. 2011;25(Apr 1):404–10.
Stander VA, Thomsen CJ, Highfill-McRoy RM. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clin Psychol Rev. 2014;34:87–98.
Funding
The study was supported by ZonMw, the Netherlands organization for Health Research and Development (40-00812-98-10041), and by a grant from the Academic Medical Center Research Council (110614) (Miranda Olff); NIMH K01 MH118428-01 (BS-J); NARSAD 27040 (XZ); R01 MH105355 (YN); RO1 MH111671 and VISN6 MIRECC (RAM); Grant 01J05415 from the Special Research Fund (BOF) at Ghent University (SCM); K01 MH118467, Julia Kasparian Fund for Neuroscience Research (LAML); R21 MH112956, R01 MH119227, McLean Hospital Trauma Scholars Fund, Barlow Family Fund, Julia Kasaparian Fund for Neuroscience Research (MLK); The Natural Science Foundation of Jiangsu Province (No. BK20221554), and the Foundation for the Social Development Project of Jiangsu (No. BE2022705) (RQ); grant no. AZV NV18-7 04-00559 from the Ministry of Health of the Czech Republic, (PŘ); NIH U54 EB020403, R01 MH116147, R01 MH129742 (CRKC); R01 MH111671, R01 MH117601, R01 AG059874, MJFF 14848 (NJ); Department of Defense award number W81XWH-12-2-0012; ENIGMA was also supported in part by NIH U54 EB020403 from the Big Data to Knowledge (BD2K) program, R56 AG058854, R01 MH116147, R01 MH111671, and P41 EB015922 (PMT); funding from the SAMRC Unit on Risk & Resilience in Mental Disorders (DJS); the South African Research Chairs Initiative in Posttraumatic Stress Disorder through the Department of Science and Technology and the National Research Foundation (SS); the South African Medical Research Council for the “Shared Roots” Flagship Project, Grant no. MRC-RFA-IFSP-01-2013/SHARED ROOTS” through funding received from the South African National Treasury under its Economic Competitiveness and Support Package (SdP); funding by the South African Medical Research Council through its Division of Research Capacity Development under the SAMRC CLINICIAN RESEARCHER (M.D PHD) SCHOLARSHIP PROGRAMME from funding received from the South African National Treasury (LLvdH); the National Natural Science Foundation of China (No. U21A20364 and No. 31971020), the Key Project of the National Social Science Foundation of China (No. 20ZDA079), the Key Project of Research Base of Humanities and Social Sciences of Ministry of Education (No.16JJD190006), and the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (No. E2CX4115CX) (LW); German Research Foundation grant to JKD (numbers DA 1222/4-1 and WA 1539/8-2) (JKD, AS, AM, HW); R01 MH113574 (IL); VA RR&D 1IK2RX000709 (NDD); VA RR&D I01RX000622; CDMRP W81XWH-08–2–0038 (SRS); VA RR&D 1K1RX002325; 1K2RX002922 (SGD); German Research Society (Deutsche Forschungsgemeinschaft, DFG; SFB/TRR 58: C06, C07) (TS, DH); Dana Foundation (to JBN); the University of Wisconsin Institute for Clinical and Translational Research (to Dr. Emma Seppala); a National Science Foundation Graduate Research Fellowship (to DWG); the National Institute of Mental Health (NIMH) R01-MH043454 and T32-MH018931 (to RJD); and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30-HD003352); R01 MH106574 (CL & TAdeR-C); VA CSR&D 1IK2CX001680; VISN17 Center of Excellence Pilot funding (EMG, GM, SMN); VA National Center for PTSD; The Beth K and Stuart Yudofsky Chair in the Neuropsychiatry of Military Post Traumatic Stress Syndrome (CGA); R21 MH102634 (IL); R01 MH105535 (IR);Department of Veterans Affairs via support for the National Center for PTSD, NIAAA via its support for (P50) Center for the Translational Neuroscience if Alcohol, and NCATS via its support of (CTSA) Yale Center for Clinical Investigation (JHK); and R01AG067103 (ASotiras). The content of this article is the sole responsibility of the authors and does not necessarily reflect the position, policy or official views of the Department of Veterans Affairs, the U.S. Government, the South African Medical Research Council, or any other funding sources listed here.
Author information
Authors and Affiliations
Contributions
Conceptualization: JY, AH, DS, RAM, and AS; methodology: JY, AH, DS, RAM, and AS; formal analysis: JY; resources:, CD, CCH, DJV, JLF, MO, MvZ, SBJK, LN, BS-J, XZ, YN, ARH, SCM, JTB, LAML, MLK, RQ, GML, PŘ, IR, ELD, CRKC, LES, NJ, PMT, DJS, SK, JCI, SS, SdP, LLvdH, LW, YZ, GL, AS, AM, HW, JKD, CS, JIH, IL, AK, MA, NDD, SRS, SGD, TS, DH, DWG, JBN, RJD, CL, TAdeR-C, JUB, BOO, EMG, GM, RAM, AS; writing—original draft: JY, AH, RAM, and AS; writing – review and edition: all authors; visualizations: JY, and AS; supervision: RAM and AS.
Corresponding author
Ethics declarations
Competing interests
LAML reports unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation (ISSTD), and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. ISSTD and NIMH were not involved in the analysis or preparation of the manuscript. CRKC received partial research support from Biogen, Inc. (Boston, USA) for research unrelated to the content of this manuscript. NJ received partial research support from Biogen, Inc. (Boston, USA) for research unrelated to the content of this manuscript. PMT received partial research support from Biogen, Inc. (Boston, USA) for research unrelated to the topic of this manuscript. RJD is the founder and president of, and serves on the board of directors for, the non-profit organization Healthy Minds Innovations, Inc. CGA has served as a consultant, speaker and/or on advisory boards for Aptinyx, FSV7, Lundbeck, Psilocybin Labs, Genentech, and Janssen; served as editor of Chronic Stress for Sage Publications, Inc; and filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). JHK is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; is a stockholder in Biohaven Pharmaceuticals; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, US Patent No. 5,447,948 (issued September 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014); has filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on March 5, 2014); US application or Patent Cooperation Treaty international application No. 14/306,382 (filed on June 17, 2014); and has filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). ASotiras holds equity in TheraPanacea and has received personal compensation for serving as a grant reviewer with the BrightFocus Foundation. The remaining authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, J., Huggins, A.A., Sun, D. et al. Examining the association between posttraumatic stress disorder and disruptions in cortical networks identified using data-driven methods. Neuropsychopharmacol. 49, 609–619 (2024). https://doi.org/10.1038/s41386-023-01763-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01763-5