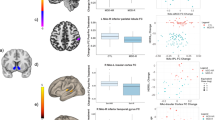
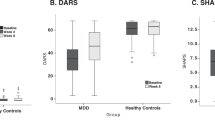
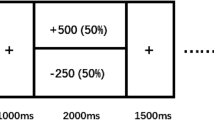
Abstract
Leading professional health bodies have called for the wider adoption of Patient Reported Outcome Measures, such as quality of life, in research and clinical practice as a means for understanding why the global burden of depression continues to climb despite increased rates of treatment use. Here, we examined whether anhedonia—an often recalcitrant and impairing symptom of depression—along with its neural correlates, was associated with longitudinal changes in patient-reported quality of life among individuals seeking treatment for mood disorders. We recruited 112 participants, including n = 80 individuals with mood disorders (58 unipolar, 22 bipolar) and n = 32 healthy controls (63.4% female). We assessed anhedonia severity along with two electroencephalographic markers of neural reward responsiveness (scalp-level ‘Reward Positivity’ amplitude and source-localized reward-related activation in the dorsal anterior cingulate cortex), and assessed quality of life at baseline, 3- and 6-month follow-up. Anhedonia emerged as a robust correlate of quality of life cross-sectionally and longitudinally among individuals with mood disorders. Furthermore, increased neural reward responsiveness at baseline was associated with greater improvements in quality of life over time, and this improvement was mediated by longitudinal improvements in anhedonia severity. Finally, differences in quality of life observed between individuals with unipolar and bipolar mood disorders were mediated by differences in anhedonia severity. Our findings indicate that anhedonia and its reward-related neural correlates are linked to variability in quality of life over time in individuals with mood disorders. Treatments capable of improving anhedonia and normalizing brain reward function may be necessary for improving broader health outcomes for individuals seeking treatment for depression.
ClinicalTrials.gov identifier: NCT01976975
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Kraus C, Kadriu B, Lanzenberger R, Zarate CA, Kasper S. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 2019;9:1–17.
Ormel J, Hollon SD, Kessler RC, Cuijpers P, Monroe SM. More treatment but no less depression: The treatment-prevalence paradox. Clin Psychol Rev. 2022;91:102111.
Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, et al. Increase in work productivity of depressed individuals with improvement in depressive symptom severity. Am J Psychiatry. 2013;170:633–41.
Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry. 2017;44:1–8.
Berwick D, Black N, Cullen D, et al. Recommendations to OECD ministers of health from the high level reflection group on the future of health statistics: strengthening the international comparison of health system performance through patient-reported indicators. Organisation for Economic Co-operation and Development. January 2017. Accessed April 22, 2022. https://www.oecd.org/health/Recommendations-from-high-level-reflection-group-on-the-future-of-health-statistics.pdf.
WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–9.
Riley WT, Pilkonis P, Cella D. Application of the National Institutes of Health patient-reported outcomes measurement information system (PROMIS) to mental health research. J Mental Health Policy Econ. 2011;14:201–8.
Calvert M, Kyte D, Price G, Valderas JM, Hjollund NH. Maximising the impact of patient reported outcome assessment for patients and society. BMJ. 2019;364:k5267.
Pizzagalli DA. Anhedonia: Preclinical, Translational, and Clinical Integration. Switzerland AG: Springer Nature; 2022.
Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, et al. Anhedonia is associated with suicidal ideation independently of depression: A meta‐analysis. Depress Anxiety. 2018;35:382–92.
Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, et al. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Exp Clin Psychopharmacol. 2010;18:562–9.
Willame H, Wacquier B, Point C, Dosogne M, Al Faker M, Loas G, et al. The association between type 2 diabetes and anhedonic subtype of major depression in hypertensive individuals. J Clin Hypertens. 2022;24:156–66.
Shaw SR, El-Omar H, Ramanan S, Piguet O, Ahmed RM, Whitton AE, et al. Anhedonia in semantic dementia—exploring right hemispheric contributions to the loss of pleasure. Brain Sci. 2021;11:998.
Chevance A, Ravaud P, Tomlinson A, Le Berre C, Teufer B, Touboul S, et al. Identifying outcomes for depression that matter to patients, informal caregivers, and health-care professionals: qualitative content analysis of a large international online survey. Lancet Psychiat. 2020;7:692–702.
McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment–resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–11.
Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–80.
Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ. Treatment for anhedonia: A neuroscience driven approach. Depress Anxiety. 2016;33:927–38.
Siddiqi SH, Haddad N, Fox MD. Circuit-targeted neuromodulation for anhedonia. Curr Top Behav Neurosci 2022. https://doi.org/10.1007/7854_2022_1350.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51.
Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005; 57:319–27.
Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clin Psychol Rev. 2014;34:417–27.
Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J Jr, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020;26:760–8.
Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Anhedonia reduction and the association between left ventral striatal reward response and 6-month improvement in life satisfaction among young adults. JAMA Psychiatry. 2019;76:958–65.
Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9.
Whitton AE, Kumar P, Treadway MT, Rutherford AV, Ironside ML, Foti D, et al. Mapping disease course across the mood disorder spectrum through a research domain criteria framework. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:706–15.
Foti D, Weinberg A, Dien J, Hajcak G. Event‐related potential activity in the basal ganglia differentiates rewards from nonrewards: response to commentary. Hum Brain Mapp. 2011;32:2267–9.
Iturra-Mena AM, Kangas BD, Luc OT, Potter D, Pizzagalli DA. Electrophysiological signatures of reward learning in the rodent touchscreen-based Probabilistic Reward Task. Neuropsychopharmacology. 2023;48:700–9.
Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol. 2009;81:1–8.
Whitton AE, Kakani P, Foti D, Van't Veer A, Haile A, Crowley DJ, et al. Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:87–95.
Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81.
Michelini G, Perlman G, Tian Y, Mackin DM, Nelson BD, Klein DN, et al. Multiple domains of risk factors for first onset of depression in adolescent girls. J Affect Disord. 2021;283:20–29.
Tsypes A, Owens M, Gibb BE. Blunted neural reward responsiveness in children with recent suicidal ideation. Clin Psychol Sci. 2019;7:958–68.
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59–65.
Klawohn J, Brush C, Hajcak G. Neural responses to reward and pleasant pictures prospectively predict remission from depression. J Abnorm Psychol. 2021;130:702–12.
First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition: NY: SCID-I/P New York; 2002.
Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78:490–8.
Ware J. SF-36 Health Survey: Manual and Interpretation Guide. Health Institute, New England Medical Center; 1993.
Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: Health Assessment Lab; 1994.
Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–6.
Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14.
Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12.
RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL. 2022: http://www.rstudio.com/.
Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: series B (Methodological). 1995;57:289–300.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
McDonald JH. Handbook of biological statistics, vol. 2. Baltimore, MD: Sparky house publishing; 2009.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications; New York, NY; 2017.
Pizzagalli DA. Toward a better understanding of the mechanisms and pathophysiology of anhedonia: Are we ready for translation? Am J Psychiatry. 2022;179:458–69.
Acknowledgements
We would like to acknowledge Thilo Deckersbach, Andrew Nierenberg, and Amy Farabaugh for facilitating recruitment of participants through the Depression Clinic and Research Program and the Bipolar Clinic and Research Program at Massachusetts General Hospital, as well as Daniel Ju Hyung Kim, Emily E. Bernstein, and Margaret E. Gigler for their assistance with patient screening and data collection at these two clinics. We would also like to thank Madeline M. Alexander, Laurie A. Scott, Nancy Hall-Brooks, and David J. Crowley for their important contributions to the screening and clinical assessment of participants recruited through the McLean Hospital Center for Depression, Anxiety and Stress Research.
Funding
This work was funded by R01MH101521 and R37MH068376 (to DAP). AEW was supported by an Investigator Grant from the National Health and Medical Research Council of Australia (GNT2017521).
Author information
Authors and Affiliations
Contributions
DAP, AEW and MTT contributed to the study conception and design; AEW, AVR, and MLI contributed to acquisition of the data; AEW, PK, DF and FD contributed to data analyses; AEW, GF and DAP contributed to statistical analyses and were responsible for data interpretation. AEW drafted the manuscript, and all authors critically reviewed the manuscript and made important intellectual contributions. DAP secured funding and provided overall supervision for the project.
Corresponding author
Ethics declarations
Competing interests
Over the past 3 years, DAP has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka, Sage Therapeutics, Sunovion, and Takeda; he has received honoraria from the Psychonomic Society and American Psychological Association (for editorial work) and Alkermes; he has received research funding from the Bird Foundation, Brain and Behavior Research Foundation, Dana Foundation, Millennium Pharmaceuticals, National Institute of Mental Health (NIMH), and Wellcome Leap (Multi-Channel Psych); he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics, and Neuroscience Software. DAP has a financial interest in Neumora Therapeutics (former BlackThorn Therapeutics), which has licensed the copyright to the Probabilistic Reward Task through Harvard University. DAP’s interests were reviewed and are managed by McLean Hospital and Massachusetts General Brigham in accordance with their conflict-of-interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. In the past 3 years, Michael Treadway has served as a paid consultant for Neumora Therapeutics (formerly BlackThorn Therapeutics) and Boehringer Ingelheim. All other authors report no financial relationships with commercial interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Whitton, A.E., Kumar, P., Treadway, M.T. et al. Distinct profiles of anhedonia and reward processing and their prospective associations with quality of life among individuals with mood disorders. Mol Psychiatry 28, 5272–5281 (2023). https://doi.org/10.1038/s41380-023-02165-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02165-1