Abstract
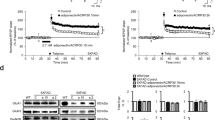
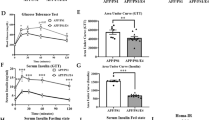
Circulating adiponectin (APN) levels decrease with age and obesity. On the other hand, a reduction in APN levels is associated with neurodegeneration and neuroinflammation. We previously showed that aged adiponectin knockout (APN−/−) mice developed Alzheimer’s like pathologies, cerebral insulin resistance, and cognitive impairments. More recently, we also demonstrated that APN deficiency increased Aβ-induced microglia activation and neuroinflammatory responses in 5xFAD mice. There is compelling evidence that deregulated insulin activities or cerebral insulin resistance contributes to neuroinflammation and Alzheimer’s disease (AD) pathogenesis. Here, we demonstrated that APN levels were reduced in the brain of AD patients and 5xFAD mice. We crossbred 5xFAD mice with APN−/− mice to generate APN-deficient 5xFAD (5xFAD;APN−/−). APN deficiency in 5xFAD mice accelerated amyloid loading, increased cerebral amyloid angiopathy, and reduced insulin-signaling activities. Pharmacokinetics study demonstrated adipoRon (APN receptor agonist) was a blood–brain barrier penetrant. AdipoRon improved neuronal insulin-signaling activities and insulin sensitivity in vitro and in vivo. Chronic adipoRon treatment improved spatial memory functions and significantly rescued neuronal and synaptic loss in 5xFAD and 5xFAD;APN−/− mice. AdipoRon lowered plaque and Aβ levels in AD mice. AdipoRon also exerted anti-inflammatory effects by reducing microglial and astrocytes activation as well as suppressing cerebral cytokines levels. The microglial phagocytic activity toward Aβ was restored after adipoRon treatment. Our results indicated that adipoRon exerts multiple beneficial effects providing important therapeutic implications. We propose chronic adipoRon administration as a potential treatment for AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zahs KR, Ashe KH. β-Amyloid oligomers in aging and alzheimer’s disease. Front Aging Neurosci. 2013;5:1–5.
Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–41.
Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY. et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128:679–89.
Talbot K, Wang H, Kazi H, Han L, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is assocaited with IGF-1 resisitance, IRS-1 dysregulation, and cogntive decline. J Clin Investig. 2012;122:1316–38.
DaRocha-Souto B, Coma M, Pérez-Nievas BG, Scotton TC, Siao M, Sánchez-Ferrer P, et al. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol Dis. 2012;45:425–37.
Ly PTT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Investig. 2013;123:224–35.
Rodríguez M, Clarimón J, Gich I, Sánchez-Ferrer P, Serenó L, Lleó A, et al. A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–67.
Bomfim TR, Forny-germano L, Sathler LB, Brito-moreira J, Houzel J, Decker H, et al. An anti-diabetes agent protects the mousebrain from defective insulin signalingcaused by Alzheimer’s disease–associated Aβ oligomers. J Clin Investig. 2012;122:1339–53.
Kondo H, Shimomura L, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–8.
Ng RCL, Chan KH. Potential neuroprotective effects of adiponectin in Alzheimer’s disease. Int J Mol Sci. 2017;18:1–13.
Une K, Takei Ya, Tomita N, Asamura T, Ohrui T, Furukawa K, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur J Neurol. 2011;18:1006–9.
Teixeira AL, Diniz BS, Campos AC, Miranda AS, Rocha NP, Talib LL, et al. Decreased levels of circulating adiponectin in mild cognitive impairment and alzheimer’s disease. NeuroMolecular Med. 2013;15:115–21.
García-Casares N, García-Arnés JA, Rioja J, Ariza MJ, Gutiérrez A, Alfaro F, et al. Alzheimer’s like brain changes correlate with low adiponectin plasma levels in type 2 diabetic patients. J Diabetes Complicat. 2016;30:281–6.
Himbergen TM van, Alexa SB, Ai M, Seshadri S, Otokozawa S, Au R. et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease results from the Framingham Heart Study. Arch Neurol. 2012;69:564–600.
Waragai M, Adame A, Trinh I, Sekiyama K, Takamatsu Y, Une K, et al. Possible Involvement of adiponectin, the anti-diabetes molecule, in the pathogenesis of Alzheimer’s disease. J Alzheimer’s Dis. 2016;52:1453–9.
Ng RC-L, Cheng OY, Kwan JSC, Ho PWL, Cheng KKY, Yeung PKK, et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments through AMPK inactivation and cerebral insulin resistance in aged mice. Mol Neurodegener. 2016;11:1–16.
Jian M, Kwan JSC, Bunting M, Ng RCL, Chan KH. Adiponectin suppresses amyloid-β oligomer (AβO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-κB signaling pathway. J Neuroinflammation. 2019;16:1–19.
Kim MW, Abid N, bin, Jo MH, Jo MG, Yoon GH, Kim MO. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci Rep. 2017;7:12435.
Zhang D, Wang X, Wang B, Garza JC, Fang X, Wang J, et al. Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Mol Psychiatry. 2017;22:1044–55.
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–9.
Gay A-S, Maroteaux L, Heurteaux C, Chabry J, Bayer P, Béchade C, et al. Adiporon, an adiponectin receptor agonist acts as an antidepressant and metabolic regulator in a mouse model of depression. Transl Psychiatry. 2018;8:159.
Patel H, Hodges AK, Curtis C, Lee SH, Troakes C, Dobson RJB, et al. Transcriptomic analysis of probable asymptomatic and symptomatic alzheimer brains. Brain Behav Immun. 2019;80:644–56.
Chu LW, Shea YF, Ha J. Validation of AD-CSF-Index in Chinese patients with Alzheimer’s disease and nondemented controls. Am J Alzheimers Dis Other Demen. 2015;30:522–6.
Schumann-Bard P, Leger M, Quiedeville A, Bouet V, Boulouard M, Freret T, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–7.
Oliveira VC, Carrara RCV, Simoes DLC, Saggioro FP, Carlotti CG, Covas DT, et al. Sudan black B treatment reduces autofluorescence and improves resolution of in situhybridization specific fluorescent signals of brain sections. Histol Histopathol. 2010;25:1017–24.
Chan KH, Lam KSL, Cheng OY, Kwan JSC, Ho PWL, Cheng KKY, et al. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS ONE. 2012;7:e52354.
Lian H, Roy E, Zheng H. Microglial phagocytosis assay. Bio-Protocols. 2016;6:1–12.
Eimer Wa, Vassar R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol Neurodegener. 2013;8:2.
Marschner A, Vervliet B, Kalisch R, Buchel C, Vansteenwegen D. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6.
Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, et al. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31:5589–95.
Grillo C, Piroli G, Lawrence R, Wrighten S, Green A, Wilson S, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–36.
Minami SS, Min S-W, Krabbe G, Wang C, Zhou Y, Asgarov R, et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat Med. 2014;20:1157–64.
Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, et al. GSK3 inhibitors show benefits in an Alzheimer’s disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol Dis. 2009;33:193–206.
Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, et al. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int J Neuropsychopharmacol. 2012;15:77–90.
Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, et al. Clearance mechanisms of Alzheimer’s amyloid-Β peptide: Implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2009;14:469–86.
Carpentier M, Robitaille Y, DesGroseillers L, Boileau G, Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:849–56.
Pérez A, Morelli L, Cresto JC, M. CE. Degradation of soluble amyloid beta-peptides 1–40, 1–42, and the Dutch variant 1–40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem Res. 2000;25:247–55.
Stanley M, Macauley SL, Holtzman DM. Changes in insulin and insulin signaling in Alzheimer’s disease: cause or consequence? J Exp Med. 2016;213:jem.20160493.
Masaki T, Anan F, Shimomura T, Fujiki M, Saikawa T, Yoshimatsu H. Association between hippocampal volume and serum adiponectin in patients with type 2 diabetes mellitus. Metabolism. 2012;61:1197–200.
Cisternas P, Martinez M, Ahima RS, William WG, Inestrosa NC. Modulation of glucose metabolism in hippocampal neurons by adiponectin and resistin. Mol Neurobiol. 2018. https://doi.org/10.1007/s12035-018-1271-x.
Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol. 2015;78:284–94.
Hsieh C-Y, Lu C-H, Yang C-Y, Ou H-T, Li C-Y. Lower risk of dementia with pioglitazone, compared with other second-line treatments, in metformin-based dual therapy: a population-based longitudinal study. Diabetologia. 2017;61:562–73.
Forny-Germano L, De Felice FG, Vieira MN, do N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front Neurosci. 2019;12:1–19.
Shah SA, Yoon GH, Chung SS, Abid MN, Kim TH, Lee HY, et al. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol Psychiatry. 2017;22:407–16.
Song J, Choi SM, Whitcomb DJ, Kim BC. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017;8:1–13.
Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131:659–85.
Charlton A, Smith EE, Hogan DB, Haffenden A, Batool S, Goodyear B, et al. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke. 2016;47:2010–6.
Guyon A, Petit-Paitel A, Cazareth J, Zarif H, Chabry J, Heurteaux C, et al. Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-κB signaling pathway. Front Cell Neurosci. 2017;11:352.
Song J, Choi S-M, Kim BC. Adiponectin regulates the polarization and function of microglia via PPAR-γ signaling under amyloid β toxicity. Front Cell Neurosci. 2017;11:64.
Min J, Ng RC-L, Chan K-H. Adiponectin suppresses amyloid-β (Aβ)-induced neuroinflammation in Alzheimer’s disease via AMPK-NF-kB singaling pathway. Soc Neurosci. 2017;126:19.
Price Ka, Varghese M, Sowa A, Yuk F, Brautigam H, Ehrlich ME, et al. Altered synaptic structure in the hippocampus in a mouse model of Alzheimer’s disease with soluble amyloid-β oligomers and no plaque pathology. Mol Neurodegener. 2014;9:41.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:1–23.
Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–7.
Mishra A, Kim HJ, Shin AH, Thayer SA. Synapse loss induced by interleukin-1β requires pre-and post-synaptic mechanisms. J Neuroimmune Pharmacol. 2012;7:571–8.
Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzumura A. Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis. 2013;4:1–8.
Kokiko-Cochran ON, Weick JP, Xu G, Ransohoff RM, Staugaitis SM, Lamb BT, et al. Microglial derived tumor necrosis factor-α drives Alzheimer’s disease-related neuronal cell cycle events. Neurobiol Dis. 2013;62:273–85.
Zhang D, Wang X, Lu XY. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology. 2016;157:2853–69.
Yau S-Y, Li A, Hoo RLC, Ching YP, Christie BR, Lee TMC, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA. 2014;111:15810–5.
Liu J, Guo M, Zhang D, Cheng S-Y, Liu M, Ding J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;109:12248–53.
Acknowledgements
We thank Prof. Kiren Rockenstein (Salk Institute) in sharing the immortalized mouse hippocampal HT-22 neuronal cell line. We also thank the technical staffs in the Faculty Core Facility, HKU, in assisting the confocal microscopy and IMARIS software operation. We are also grateful to the Brain Bank, NIHR BRC at Imperial College and Alzheimer’s Research, to provide human paraffin sections and human CSF. This work was supported by grants to RC-LN from the Health & Medical Research Fund (ref no. 03143856) and Chan Kin Shing Charitable Trust to K-HC. This work was also partly supported by grants to ML from the Hong Kong General Research Fund (GRF/HKBU12101417, GRF/HKBU12100618) and the Health & Medical Research Fund (HMRF/15163481, HMRF14150811). This work was also partly supported by grants to S-SKD from the Hong Kong Innovation Technology Fund (ITS/253/14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ng, R.CL., Jian, M., Ma, O.KF. et al. Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer’s disease mouse model. Mol Psychiatry 26, 5669–5689 (2021). https://doi.org/10.1038/s41380-020-0701-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0701-0
This article is cited by
-
Liver-specific adiponectin gene therapy suppresses microglial NLRP3-inflammasome activation for treating Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Adiponectin deficiency accelerates brain aging via mitochondria-associated neuroinflammation
Immunity & Ageing (2023)
-
Advances in Molecular Psychiatry – March 2023: mitochondrial function, stress, neuroinflammation – bipolar disorder, psychosis, and Alzheimer’s disease
Molecular Psychiatry (2023)
-
Cellular and Molecular Regulation of Exercise—A Neuronal Perspective
Cellular and Molecular Neurobiology (2023)
-
Adiponectin suppresses tumor growth of nasopharyngeal carcinoma through activating AMPK signaling pathway
Journal of Translational Medicine (2022)