Abstract
Background
Acid-suppressing medications (ASMs) are commonly prescribed in the neonatal intensive care unit (NICU), in particular among preterm infants, despite well-established adverse effects and little evidence to support efficacy.
Local problem
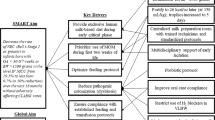
We sought to develop an initiative to reduce ASM exposure in our predominantly inborn level III NICU. Our specific aim was to reduce the number of nonindicated ASM prescriptions by 50% within a 12-month period.
Methods
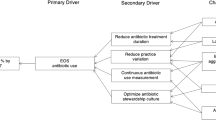
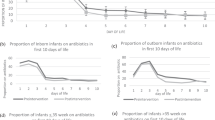
Our multidisciplinary team developed an evidence-based guideline defining indications for ASM prescription in a level III NICU. Plan-do-study-act cycles included staff education, formal clinical practice guideline implementation, and implementation of standardized documentation tools in the electronic health record (EHR). Outcome measures were the number of nonindicated and total inpatient prescriptions started per month, duration of ASM prescription, and number of prescriptions continued after NICU discharge. Balancing measures were the number of patients started on thickened feeds and number of patients discharged home on nasogastric tube feeds. We used statistical process control and Pareto charts to assess these measures over a 12-month baseline period, 9-month implementation period, and 19-month post-implementation period spanning September 2017–December 2020.
Results
Nonindicated ASM prescriptions decreased from median 3 to 0 per month from the baseline to post-implementation period. Simultaneously, the median number of ASM prescriptions at discharge declined from 2 to 0 per month. The median duration of inpatient prescriptions declined from 23 to 7 days. Rates of patients started on thickened feeds and patients discharged home on nasogastric tube feeds remained stable throughout the study.
Conclusion
Enactment of an evidence-based guideline was associated with a substantial decline in nonindicated ASM use in our NICU and a decline in duration of exposure to ASM’s when prescribed. Our interventions proved effective in altering clinical practice and could be applied to other NICUs with similar patient populations aiming to reduce ASM use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hassall E. Over-prescription of acid-suppressing medications in infants: how it came about, why it’s wrong, and what to do about it. J Pediatr. 2012;160:193–198.
Ewer AK, Durbin GM, Morgan ME, Booth IW. Gastric emptying and gastro-oesophageal reflux in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F117–121.
Eichenwald EC, Committee on Fetus and Newborn. Diagnosis and management of gastroesophageal reflux in preterm infants. Pediatrics. 2018;142. https://doi.org/10.1542/peds.2018-1061.
Slocum C, Arko M, Di Fiore J, Martin RJ, Hibbs AM. Apnea, bradycardia and desaturation in preterm infants before and after feeding. J Perinatol J Calif Perinat Assoc. 2009;29:209–212.
Angelidou A, Bell K, Gupta M, Tropea Leeman K, Hansen A. Implementation of a guideline to decrease use of acid-suppressing medications in the NICU. Pediatrics. 2017;140. https://doi.org/10.1542/peds.2017-1715.
Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Neonatal histamine-2 receptor antagonist and proton pump inhibitor treatment at United States Children’s Hospitals. J Pediatr. 2016;174:63–70.e3.
D’Agostino JA, Passarella M, Martin AE, Lorch SA. Use of gastroesophageal reflux medications in premature infants after NICU discharge. Pediatrics. 2016; 138. https://doi.org/10.1542/peds.2016-1977.
Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154:514–520.e4.
Omari TI, Haslam RR, Lundborg P, Davidson GP. Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J Pediatr Gastroenterol Nutr. 2007;44:41–44.
Moore DJ, Tao BS-K, Lines DR, Hirte C, Heddle ML, Davidson GP. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr. 2003;143:219–223.
López-Alonso M, Moya MJ, Cabo JA, Ribas J, del Carmen Macías M, Silny J, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006;118:e299–308.
Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–51.
Poets CF. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics. 2004;113:e128–132.
Wheatley E, Kennedy KA. Cross-over trial of treatment for bradycardia attributed to gastroesophageal reflux in preterm infants. J Pediatr. 2009;155:516–521.
Hyman PE, Everett SL, Harada T. Gastric acid hypersecretion in short bowel syndrome in infants: association with extent of resection and enteral feeding. J Pediatr Gastroenterol Nutr. 1986;5:191–197.
Hagander L, Muszynska C, Arnbjornsson E, Sandgren K. Prophylactic treatment with proton pump inhibitors in children operated on for oesophageal atresia. Eur J Pediatr Surg. 2012;22:139–142.
Venkatesan NN, Pine HS, Underbrink M. Laryngopharyngeal reflux disease in children. Pediatr Clin North Am. 2013;60:865–878.
Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst Rev. 2014;CD008550.
Pai AK, Fox VL. Gastrointestinal bleeding and management. Pediatr Clin North Am. 2017;64:543–561.
Rosen R, Mitchell PD, Amirault J, Amin M, Watters K, Rahbar R. The edematous and erythematous airway does not denote pathologic gastroesophageal reflux. J Pediatr. 2017;183:127–131.
Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–142.
More K, Athalye-Jape G, Rao S, Patole S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants. Am J Perinatol. 2013;30:849–856.
Bianconi S, Gudavalli M, Sutija VG, Lopez AL, Barillas-Arias L, Ron N. Ranitidine and late-onset sepsis in the neonatal intensive care unit. J Perinat Med. 2007;35:147–150.
Romaine A, Ye D, Ao Z, Fang F, Johnson O, Blake T, et al. Safety of histamine-2 receptor blockers in hospitalized VLBW infants. Early Hum Dev. 2016;99:27–30.
Malchodi L, Wagner K, Susi A, Gorman G, Hisle-Gorman E. Early acid suppression therapy exposure and fracture in young children. Pediatrics. 2019;144. https://doi.org/10.1542/peds.2018-2625.
Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172:e180315.
Wang Y-H, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Association between proton pump inhibitor use and risk of asthma in children. JAMA Pediatr. 2021;175:394–403.
Ho T, Dukhovny D, Zupancic JAF, Goldmann DA, Horbar JD, Pursley DM. Choosing wisely in newborn medicine: five opportunities to increase value. Pediatrics. 2015;136:e482–489.
Reinhart RM, McClary JD, Zhang M, Marasch JL, Hibbs AM, Nock ML. Reducing antacid use in a level IV NICU: a QI project to reduce morbidity. Pediatr Qual Saf. 2020;5:e303.
Gupta M, Kaplan HC. Measurement for quality improvement: using data to drive change. J Perinatol. 2020;40:962–971.
Larson EB. N-of-1 clinical trials. A technique for improving medical therapeutics. West J Med. 1990;152:52–56.
Levy EI, Salvatore S, Vandenplas Y, de Winter JP. Prescription of acid inhibitors in infants: an addiction hard to break. Eur J Pediatr. 2020;179:1957–1961.
Jadcherla SR, Hasenstab KA, Wei L, Osborn EK, Viswanathan S, Gulati IK, et al. Role of feeding strategy bundle with acid-suppressive therapy in infants with esophageal acid reflux exposure: a randomized controlled trial. Pediatr Res. 2021;89:645–652.
Funding
No funding was received for this project.
Author information
Authors and Affiliations
Contributions
JDT, SER, and KAB contributed to the design and methods for the project. JDT and SER contributed to data retrieval. JDT contributed to the display and statistical analysis of the data. JDT and KAB contributed to the analysis and interpretation of the data, as well as initial drafting of the manuscript. All authors contributed to critical revision of the manuscript prior to publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thai, J.D., Rostas, S.E., Erdei, C. et al. A quality improvement initiative to reduce acid-suppressing medication exposure in the NICU. J Perinatol 42, 1118–1125 (2022). https://doi.org/10.1038/s41372-021-01262-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01262-9