Abstract
Objective
Building on our previous study, showing a correlation between ferritin in serum and urine, we conducted a feasibility evaluation, measuring urinary ferritin as a potential noninvasive screening test for iron deficiency among NICU patients.
Study design
This was a prospective analysis of paired serum/urine ferritin levels. We defined iron-limited erythropoiesis by a RET-He <5th percentile lower reference interval (<28 pg).
Results
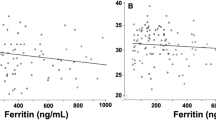
We obtained 49 paired serum/urine samples from neonates judged as at-risk for iron deficiency. Urine ferritin (“corrected” for urine creatinine and specific gravity) correlated with serum ferritin (correlation coefficient of log10-transformed values 0.44). A corrected urine ferritin <12 ng/mL had a sensitivity of 82% (95% CI, 67–93%) and a specificity of 100% (CI, 66–100%) for detecting iron-limited erythropoiesis, with a positive predictive value of 100% (CI, 89–100%).
Conclusions
Measuring urinary ferritin in NICU patients is feasible. Since low values identify iron-limitation, this could become a useful noninvasive screen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cusick SE, Georgieff MK, Rao R. Approaches for reducing the risk of early-life iron deficiency-induced brain dysfunction in children. Nutrients. 2018;10:227.
Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr. 2017;106:1588S–93S.
Fleming RE. Cord serum ferritin levels, fetal iron status, and neurodevelopmental outcomes: Correlations and confounding variables. J Pediatr. 2002;140:145–8.
Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70.
Marell PS, Blohowiak SE, Evans MD, Georgieff MK, Kling PJ, Tran PV. Cord blood-derived exosomal CNTN2 and BDNF: potential molecular markers for brain health of neonates at risk for iron deficiency. Nutrients. 2019;11:2478.
McCarthy PJ, Zundel HR, Johnson KR, Blohowiak SE, Kling PJ. Impact of growth restriction and other prenatal risk factors on cord blood iron status in prematurity. J Pediatr Hematol Oncol. 2016;38:210–5.
MacQueen BC, Christensen RD, Ward DM, Bennett ST, O’Brien EA, Sheffield MJ, et al. The iron status at birth of neonates with risk factors for developing iron deficiency: a pilot study. J Perinatol. 2017;37:436–40.
MacQueen BC, Christensen RD, Baer VL, Ward DM, Snow GL. Screening umbilical cord blood for congenital iron deficiency. Blood Cells Mol Dis. 2019;77:95–100.
Dosch NC, Guslits EF, Weber MB, Murray SE, Ha B, Coe CL, et al. Maternal obesity affects inflammatory and iron indices in umbilical cord blood. J Pediatr. 2016;172:20–28.
Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800:760–9.
DeLoughery TG. Iron deficiency anemia. Med Clin North Am. 2017;101:319–32.
Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82.
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16.
Ishikawa K, Narita O, Saito H, Kato K. Determination of ferritin in urine and in serum of normal adults with a sensitive enzyme immunoassay. Clin Chim Acta. 1982;123:73–81.
Bahr TM, Christensen RD, Ward DM, Meng F, Jackson LK, Doyle K, et al. Ferritin in serum and urine: a pilot study. Blood Cells Mol Dis. 2019;76:59–62.
Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303–8.
Buttarello M, Rauli A, Mezzapelle G. Reticulocyte count and extended reticulocyte parameters by Mindray BC-6800: reference intervals and comparison with Sysmex XE-5000. Int J Lab Hematol. 2017;39:596–603.
Christensen RD, Henry E, Bennett ST, Yaish HM. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J Perinatol. 2016;36:61–6.
Lorenz L, Peter A, Arand J, Springer F, Poets CF, Franz AR. Reticulocyte haemoglobin content declines more markedly in preterm than in term infants in the first days after birth. Neonatology. 2017;112:246–50.
Al-Ghananim RT, Nalbant D, Schmidt RL, Cress GA, Zimmerman MB, Widness JA. Reticulocyte hemoglobin content during the first month of life in critically ill very low birth weight neonates differs from term infants, children, and adults. J Clin Lab Anal. 2016;30:326–34.
Lorenz L, Arand J, Büchner K, Wacker-Gussmann A, Peter A, Poets CF, et al. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100:F198–202.
Amin K, Bansal M, Varley N, Wang H, Amin S. Reticulocyte hemoglobin content as a function of iron stores at 35–36 weeks post menstrual age in very premature infants. J Matern Fetal Neonatal Med. 2019;32:1–6.
Buttarello M, Pajola R, Novello E, Mezzapelle G, Plebani M. Evaluation of the hypochromic erythrocyte and reticulocyte hemoglobin content provided by the Sysmex XE-5000 analyzer in diagnosis of iron deficiency erythropoiesis. Clin Chem Lab Med. 2016;54:1939–45.
Löfving A, Domellöf M, Hellström-Westas L, Andersson O. Reference intervals for reticulocyte hemoglobin content in healthy infants. Pediatr Res. 2018;84:657–61.
Lorenz L, Peter A, Arand J, Springer F, Poets CF, Franz AR. Reference ranges of reticulocyte haemoglobin content in preterm and term infants: a retrospective analysis. Neonatology. 2017;111:189–94.
Christensen RD, Jopling J, Henry E, Wiedmeier SE. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J Perinatol. 2008;28:24–28.
Urrechaga E, Borque L, Escanero JF. Potential utility of the new Sysmex XE 5000 red blood cell extended parameters in the study of disorders of iron metabolism. Clin Chem Lab Med. 2009;47:1411–6.
Urrechaga E, Borque L, Escanero JF. Percentage of hypochromic erythrocytes as a potential marker of iron availability. Clin Chem Lab Med. 2011;50:685–7.
Levy S, Schapkaitz E. The clinical utility of new reticulocyte and erythrocyte parameters on the Sysmex XN 9000 for iron deficiency in pregnant patients. Int J Lab Hematol. 2018;40:683–90.
German K, Vu PT, Grelli KN, Denton C, Lee G, Juul SE. Zinc protoporphyrin-to-heme ratio and ferritin as measures of iron sufficiency in the Neonatal Intensive Care Unit. J Pediatr. 2018;194:47–53.
German K, Vu PT, Irvine JD, Juul SE. Trends in reticulocyte hemoglobin equivalent values in critically ill neonates, stratified by gestational age. J Perinatol. 2019;39:1268–74.
McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer BA, et al. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediatr Hematol Oncol. 2013;35:473–477.
Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513–518.
Siddappa AM, Olson RM, Spector M, Northrop E, Zamora T, Brearley AM, et al. High prevalence of iron deficiency despite standardized high-dose iron supplementation during recombinant erythropoietin therapy in extremely low gestational age newborns. J Pediatr 2020;S0022-3476:30434–0.
Acknowledgements
We thank Kendell R. German, MD, University of Washington, for reviewing the paper and making helpful suggestions. We also thank Vickie L. Baer, RN, NICU data research manager, for building the RedCap program, Susan Christensen, RN, NICU research nursing, for entering patients into the study, and the families, neonatologists, and neonatal nurse practitioners of Utah Valley Hospital, Provo, Utah, for their support of this study. We also thank Ryan Cordner, PhD, Department of Microbiology & Molecular Biology, Brigham Young University, Provo, UT, and Dennis Eggett, PhD, Department of Statistics, Brigham Young University, Provo, UT for assistance and advice.
Funding
The study was supported in part by grant U54DK110858 from the US Public Health Service, and by funds from Intermountain Healthcare Women and Newborns Research (to EG), and by funds from the Department of Pediatrics (to TMB), University of Utah Health, Salt Lake City, UT.
Author information
Authors and Affiliations
Contributions
All authors reviewed and edited the paper, approved the final version, and take responsibility for the contents. The study was conceived, organized, and conducted by EG, JBB, JOE, SF, and RDC. The laboratory aspects were conceived and conducted by BAM and DMW. Data assembly, display, statistical analysis, and writing the initial paper draft, were performed by TMB, JBB, RKO, and RDC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gerday, E., Brereton, J.B., Bahr, T.M. et al. Urinary ferritin; a potential noninvasive way to screen NICU patients for iron deficiency. J Perinatol 41, 1419–1425 (2021). https://doi.org/10.1038/s41372-020-0746-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0746-6