Abstract
A titanium phosphonate cluster with the formula of [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf was synthesized. Titanium tetraisopropoxide and phenylphosphonic acid were mixed in tetrahydrofuran and subjected to hydrolysis to provide a white crystal of titanium phosphonate. The titanium phosphonate was mixed with poly(methyl methacrylate) (PMMA) to form a hybrid film. The titanium phosphonate content in the resulting mixture was <30 wt% and the obtained material was yellow. The degradation temperature increased by ~30 °C owing to the hybridization of the titanium phosphonate cluster with PMMA. In another approach, titanium phosphonate was mixed with poly(vinyl alcohol) (PVA) to form a hybrid film. The titanium phosphonate content in the mixture was <50 wt% and the obtained material was colorless. Isopropyl alcohol was formed in this reaction, suggesting the formation of a titanium phosphonate/PVA hybrid by the reaction of PVA with an isopropoxy group in titanium phosphonate.
Similar content being viewed by others
Introduction
Metal oxide clusters are unique compounds composed of metal–oxygen bonds as a main chain bearing side chains, such as organic groups, hydrides or halogens. Polysilsesquioxane cages1 are well-known compounds with silicon and oxygen atoms in the main framework. Oxo-titanium clusters2 composed of titanium and oxygen atoms are also examples of well-studied metal oxide clusters. Recently, organic–inorganic hybrid polymers prepared using these clusters were found to improve both the thermostability and wear resistivity relative to the corresponding organic polymers.3, 4, 5, 6, 7 Organic–inorganic hybrid materials are divided into two classes based on their bonding type. Class I comprises the polymers that are bonded by weak interactions, such as van der Waals force and hydrogen bonding. Class II comprises the polymers that are mixed by strong interactions, such as covalent bonding, or the ionic bonding between organic and inorganic components.8 For example, Chujo4 reported that the hybrid materials of polysulfide-bridged polyhedral oligosilsesquioxane (POSS) with poly(methyl methacrylate) (PMMA) or polystyrene (class I) showed a high thermal stability and high refractive indices compared with the calculated values.9 Schubert reported that PMMA cross-linked with Ti4O2(OiPr)6(OMc)6 (OMc=methacrylate) (class II) exhibits a high thermal stability.3, 4, 5, 6, 7 Therefore, organic–inorganic hybrid materials are very interesting because we can expect that the improvement of their physical properties will open new applications in materials science.
Clusters containing three components are being reported in current research. Titanium phosphonate clusters formed by titanium, oxygen and phosphorus have the potential for high chemical and thermostable properties owing to the presence of Ti–O–P bonds. The interactions among organophosphonate groups are easily controlled by changing the organic groups on titanium or phosphorus atoms. Organic–inorganic hybrid materials containing titanium phosphonate clusters would be prepared by an alcohol exchange reaction or the sol-gel process10 owing to the presence of an alkoxy group on the titanium atom. Although the synthesis of titanium phosphonate clusters has been reported,11, 12, 13, 14, 15, 16 the synthesis of organic–inorganic hybrid materials containing these clusters has not been investigated. The use of dimethylsulfoxide (DMSO) as the coordinating solvent is one of the problems encountered in the previous preparation of hybrid materials using clusters. DMSO has strong coordinating properties compared with solvents, such as tetrahydrofuran (THF), which is a common coordinating solvent. Therefore, we expected that a new titanium phosphonium cluster with THF will be useful for the preparation of organic–inorganic hybrid materials because exchange reactions between THF and the ester groups of PMMA or the hydroxyl groups in poly(vinyl alcohol) (PVA) will occur easily. In this work, we report the synthesis and structure of a novel titanium phosphonate cluster, [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf (TiOPPh), shown in Scheme 1. We also prepared PMMA or PVA hybrid materials containing the cluster and investigated the properties of these materials.
Experimental Procedure
Measurements
NMR spectra were recorded using a JEOL Resonance JNM-ECP 500 spectrometer (JEOL, Akishima, Japan), (1H at 500.16 MHz, 13C at 125.77 MHz and 31P at 202.46 MHz) at 24 °C. The chemical shifts were reported in p.p.m. relative to CDCl3 used as an internal standard (for 1H: 7.26 p.p.m. in residual CHCl3, 13C: 77.16 p.p.m.) and H3PO4 as an external standard (for 31P: 0.00 p.p.m.). Infrared spectra were recorded on a JASCO FT/IR-6100 FT-IR spectrophotometer (JASCO, Hachioji, Japan) using attenuated total reflectance (ZnSe prism, JASCO ATR PRO 0450-S). Thermogravimetric-differential thermal analysis was performed using a NETZSCH JAPAN, TG-DTA 2000SE analyzer (Yokohama, Japan). The samples were heated to 1000 °C under an airflow at the rate of 10 °C per min. Transmittance spectra were recorded using a JASCO V-670 spectrophotometer equipped with an integrating-sphere photometer (JASCO ISN-470 type) in the 200–800 nm wavelength range. Refractive indices were determined using an Otsuka Electronics FE-3000 refractive film thickness monitor (Osaka, Japan).
X-ray structure analyses
For structural determination, crystal data were collected using a Bruker AXS, SMART APEX CCD X-ray diffractometer (Yokohama, Japan) equipped with a rotating-anode X-ray generator emitting graphite-monochromatic Mo-Kα radiation (λ=0.71073 Å) at 77 K. Empirical absorption corrections using equivalent reflections and Lorentzian polarization correction were performed using the SADABS program.17 All data were collected using SMART and Bruker SAINTPLUS (Version 6.45) software (Bruker AXS Inc., Madison, WI, USA). The structures were solved using the SHELXS-97 program18 and refined against F2 using SHELEXL-97.19 CCDC 1487366 contains the supplementary crystallographic data for TiOPPh. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk.
Materials
All solvents were purified by a standard process20 and stored over activated molecular sieves. Titanium tetraisopropoxide and PVA (degree of polymerization, 500) were purchased from Wako Pure Chemical Industries, Tokyo, Japan. Phenylphosphonic acid was purchased from Tokyo Chemical Industry and PMMA (Mw=997 000 g mol−1) was purchased from Sigma-Aldrich (Tokyo, Japan). All reagents were used as received.
Synthesis of TiOPPh
The synthesis reaction was carried out in an argon atmosphere. Titanium tetraisopropoxide (36 ml, 0.12 mol) was added to PhPO3H2 (9.56 g, 60.5 mmol) and H2O (0.36 ml, 20 mmol) in 48 ml THF at room temperature, with white precipitation appearing shortly afterward. After several hours of stirring, the mixture became a colorless solution, whereupon the stirring was stopped. Colorless block crystals from the solution appeared after several weeks, and were then filtrated and dried in vacuo. White block crystals were obtained in a yield of 8.25 g (34%).
1H NMR (500 MHz, CDCl3/7.26 p.p.m.): δ=1.06 (d, J=5.5 Hz, 12H), 1.37 (d, J=6.0 Hz, 18H), 1.44 (d, J=6.0 Hz, 18H), 1.75–1.81 (m, 6H), 3.79–3.82 (m, 6H), 4.69 (sept, J=6.0 Hz, 3H), 4.94 (br-s, 2H), 5.03 (sept, J=6.0 Hz, 3H), 7.30–7.38 (m, 9H), 7.88 (dd, J=7.5 Hz, 3JP–H=12.0 Hz, 6H).
13C{1H} NMR (126 MHz, CDCl3/77.16 p.p.m.): δ=24.51, 24.83, 25.36, 68.84, 78.45, 79.14, 79.40, 127.31 (d, 2JP–C=14.4 Hz), 129.72 (d, 4JP–C=2.9 Hz), 131.05 (d, 3JP–C=9.6 Hz), 134.44 (d, 1JP–C=203.9 Hz); 31P{1H} NMR (202 MHz, CDCl3/p.p.m.): δ=9.2. Ceramic yield: 40.4% (calcd. for Ti4P3O16 41.7%).
Preparation of free-standing hybrid films
PMMA–TiOPPh hybrid films
5 ml of PMMA in toluene (50 g l−1) was added to TiOPPh in toluene (31 g l−1), and the mixture was stirred at room temperature overnight. The solution was poured into a 60 mmφ glass petri dish followed by curing at 50 °C for 1 day and then at 120 °C for 1 day in an air atmosphere. The thickness of the films was ~85 μm.
PVA–TiOPPh hybrid films
0.12 g of PVA dissolved in 10 ml of DMSO was added to TiOPPh dissolved in 1 ml of THF and the mixture was stirred at room temperature overnight. The solution was poured into a 50 mm Teflon petri dish followed by curing at 50 °C for 1 day and then at 120 °C for 1 day in an air atmosphere. The thickness of the films was ~60 μm.
Preparation of hybrid thin films
A solution of 0.12 g of PVA in 5 ml of deionized water was added to TiOPPh in 5 ml of THF and the mixture was stirred at room temperature overnight. Hybrid thin films were prepared by spin-coating of the solution on a silicon wafer at 1000 r.p.m. for 20 s and then heating at 120 °C for 2 min in air.
Results and Discussion
Characterization of TiOPPh
TiOPPh was synthesized by the reaction of titanium tetraisopropoxide with PhPO3H2 and water in THF. The formation of this cluster was confirmed using various NMR spectra, Fourier transform infrared (FT-IR) spectra and single-crystal X-ray structural analysis. Single-crystal X-ray structural diffraction showed that TiOPPh is composed of four titanium atoms, three PhPO3 groups, five terminal OiPr, three bridged OiPr, one μ3-O and one THF molecule (Figure 1,Table 1). The main TiOPPh framework consisted of three bridged RPO3 between one Ti atom coordinated by THF and one Ti3O containing a μ3-O atom. Three phosphonates are arranged in one direction, one on top of the other and are capped by a THF-coordinated Ti atom. This structure is very similar to that of TiOPPh·dmso.11, 12 In the 1H NMR spectrum, three doublet signals attributed to the CH3 in the isopropoxy group were found at 1.06, 1.37 and 1.44 p.p.m. with respective integration ratios of 12H, 18H and 18H. These signals are assigned to the terminal isopropoxy group on the Ti coordinated by THF, the terminal isopropoxy group on the Ti3O unit and the bridged isopropoxy group, respectively. CH in the isopropoxy group shows signals at 4.69, 4.94 and 5.03 p.p.m. The signals derived from the phenyl group shown at 7.30–7.38 p.p.m. as a multiplet are assigned to the overlapping signals of m- and p-CH in the phenyl group, and the double doublet signal observed at 7.88 p.p.m. was assigned to o-CH in the phenyl group. The 13C NMR spectrum shows signals owing to CH3 at 24.51 and 24.83 p.p.m., owing to THF at 25.36 and 68.84 p.p.m., and owing to CH at 78.45, 79.14 and 79.40 p.p.m. The signals derived from the phenyl group appear in the doublet because of the coupling between the phosphorus and the carbon atoms. The signals observed at 127.31, 129.72, 131.05 and 134.44 p.p.m. were assigned to o-CH, p-CH, m-CH and ipso-CH, respectively. The 31P NMR spectrum shows signals at 9.26 p.p.m. in CDCl3; in C6D6, the 31P shows signals at 6.8 p.p.m. with an integrated intensity of 2 and at 7.2 p.p.m. with an integrated intensity of 1.
ORTEP drawing of TiOPPh with thermal ellipsoids at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected interatomic distances (Å) and bond angles (°) are as follows: Ti(1)–O(1) 1.972(6), Ti(1)–O(7) 1.962(4), Ti(1)–O(11) 2.010(5), Ti(1)–O(14) 1.779(5), Ti(4)–O(8) 1.924(6), Ti(4)–O(17) 1.820(7), Ti(4)–O(19) 2.162(6), P(1)–O(1) 1.536(4); O(1)–Ti(1)–O(3) 90.7(2), O(1)–Ti(1)–O(11) 87.5(2), O(7)–Ti(1)–O(14) 175.4(2), O(8)–Ti(4)–O(9) 94.5(2), O(8)–Ti(4)–O(19) 174.3(3), O(1)–P(1)–O(2) 111.4(3), O(1)–P(1)–O(8) 110.9(3). TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
The FT-IR spectrum of TiOPPh shows the following absorption bands: νC–H of phenyl at 3056 cm−1, νC–H of the isopropoxy group at 2970 and 2928 cm−1, νC–H of THF at 2862 cm−1, νC=C of phenyl at 1439 cm−1, δC–H at 1375 and 1362 cm−1, νC–C at 1156 and 1132 cm−1, νC–O and νP–O at 989–949 cm−1 overlapping with δC=C at 754 and 696 cm−1 assigned to the out-of-plane phenyl vibration, and νTi–O at 614–558 cm−1. The thermogravimetric-differential thermal analysis of TiOPPh shows weight losses at 80–250 °C (41%) assigned to the decomposition of the isopropoxy group, THF and the carbons in phenyl, as well as at 450 °C (8%) and 800 °C (10%).
Preparation of free-standing hybrid films
PMMA hybrid films were prepared from a toluene solution, and PVA hybrid films were prepared from a solution of DMSO and THF. A photograph of the hybrid films is shown in Figure 2. The PMMA films changed from colorless to yellow as the TiOPPh concentration increased. However, colorless and transparent films were prepared by using PVA. We note that we have attempted to prepare hybrid films containing 30 wt% of PMMA and 50 wt% of PVA. Unfortunately, these films were not prepared because they were too rigid to retain the form of free-standing films.
Photograph of hybrid films. (a) PMMA only, (b) PMMA-10 wt% TiOPPh, (c) PMMA-20 wt% TiOPPh, (d) PVA only, (e) PVA-10 wt% TiOPPh, (f) PVA-20 wt% TiOPPh and (g) PVA-40 wt% TiOPPh. PMMA, poly(methyl methacrylate); PVA, poly(vinyl alcohol); TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
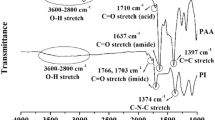
FT-IR spectra are shown in Figure 3. All PMMA hybrid films show νC–H at 3000–2950 cm−1, νC–H at 1723 cm−1, and νC–O–C at 1190 and 1144 cm−1. PMMA–TiOPPh hybrid films show new bands owing to νP=O at 1040 cm−1 and νTi–O at ~550 cm−1. However, the νP–O band could be observed only for the 20 wt% TiOPPh. The bands derived from PMMA were very similar, especially νC=O at 1723 cm−1. These results indicated that the carbonyl groups of PMMA did not coordinate to the TiOPPh Ti atom via an exchange reaction between THF and the carbonyl groups. However, νC–O–C shifted from 1144 cm−1 (PMMA) to 1142 cm−1 (PMMA–TiOPPh hybrid). Therefore, PMMA and TiOPPh can be blended by using a weak interaction between the PMMA methoxy group and TiOPPh. However, all PVA hybrid films showed νO–H at 3300 cm−1, νC–H at 2911 cm−1 and νC–C at 1421 cm−1. The signal intensity owing to νP–O at 1000 cm−1 increased and that owing to νO–H decreased with increasing amount of TiOPPh. On the basis of these results, an alcohol exchange reaction between the hydroxy group in PVA and the isopropoxy group in TiOPPh was confirmed.
FT-IR spectra of (i) PMMA–TiOPPh hybrid films: (a) PMMA only, (b) PMMA-2.5 wt% TiOPPh, (c) PMMA-10 wt% TiOPPh, (d) PMMA-20 wt% TiOPPh and (e) TiOPPh only; and (ii) PVA–TiOPPh hybrid films: (a) PVA only, (b) PVA-2.5 wt% TiOPPh, (c) PVA-10 wt% TiOPPh, (d) PVA-20 wt% TiOPPh and (e) PVA-40 wt% TiOPPh. PMMA, poly(methyl methacrylate); PVA, poly(vinyl alcohol); TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
Transmittance spectra are shown in Figure 4 and the data are summarized in Table 2. As the TiOPPh concentration increased, the transparency of PMMA hybrid films in the visible region decreased significantly. Moreover, these TiOPPh–PMMA hybrid films were yellow. However, the transparency of the PVA hybrid films was high compared with that of the PVA films. The origin of the yellow color in PMMA films is assigned to the aggregation and/or polymerization of TiOPPh caused by the water in the air. In contrast, TiOPPh in PVA appears to be highly dispersed. Therefore, the PVA hybrid films were obtained as colorless films. These conclusions are also supported by the infrared (IR) spectroscopy results.
UV–Vis transmission spectra of (i) PMMA–TiOPPh hybrid films: (a) PMMA only, (b) PMMA-2.5 wt% TiOPPh, (c) PMMA-10 wt% TiOPPh and (d) PMMA-20 wt% TiOPPh; and (ii) PVA–TiOPPh hybrid films: (a) PVA only, (b) PVA-2.5 wt% TiOPPh, (c) PVA-10 wt% TiOPPh, (d) PVA-20 wt% TiOPPh and (e) PVA-40 wt% TiOPPh. PMMA, poly(methyl methacrylate); PVA, poly(vinyl alcohol); TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf; UV–Vis, ultraviolet–visible. A full color version of this figure is available at the Polymer Journal journal online.
Thermogravimetric-differential thermal analysis thermograms of hybrid films are shown in Figure 5 and the data are summarized in Table 2. The temperatures of 10% weight loss (Td10) were 279 and 278 °C for PMMA and PVA, respectively. The Td10 values for PMMA–TiOPPh hybrid films were ~310 °C and were 30 °C higher than that of PMMA. TiOPPh improves the thermal stability of PMMA such that the weight loss is 40 wt% at 310 °C. The improvement of thermal stability was the same as that for other blend, or hybrid PMMA materials containing polysulfide-bridged POSS,9 zirconia nanocrystals modified with 3-(methacryloxy)propyl-trimethoxysilane21 and titania modified with 2-hydroxyethyl methacrylate22 that were reported in previous studies. However, the Td10 of the PVA–TiOPPh hybrid films decreased with increasing TiOPPh concentration, such that the Td10 of PVA-30 wt% of the TiOPPh hybrid film was decreased by 45 °C compared with the value obtained for PVA. The Td10 decrease can be attributed to the reaction of the isopropoxy group in TiOPPh with the residual hydroxy group in PVA.
Thermogravimetric analysis traces of (i) PMMA–TiOPPh hybrid films: (a) PMMA only, (b) PMMA-2.5 wt% TiOPPh, (c) PMMA-10 wt% TiOPPh and (d) PMMA-20 wt% TiOPPh; and (ii) PVA–TiOPPh hybrid films: (a) PVA only, (b) PVA-2.5 wt% TiOPPh, (c) PVA-10 wt% TiOPPh, (d) PVA-20 wt% TiOPPh and (e) PVA-40 wt% TiOPPh. PMMA, poly(methyl methacrylate); PVA, poly(vinyl alcohol); TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
The refractive indices of PVA-hybrid thin films at 633 nm were measured. The refractive index of PVA only was 1.488, and those of PVA-2.5 wt% TiOPPh and PVA-10 wt% TiOPPh were 1.500 and 1.501, respectively. The increase of the refractive index values supports the formation of hybrids.
Conclusion
A titanium phosphonate cluster with a formula of TiOPPh was synthesized via the reaction of titanium tetraisopropoxide and phenylphosphonic acid in water and THF. Titanium phosphonate was mixed with PMMA to form a yellow hybrid film at a concentration of <30 wt%. The degradation temperature increased by ~30 °C when the titanium phosphonate cluster was hybridized with PMMA. However, titanium phosphonate was mixed with PVA to form a hybrid film. The resulting content was <50 wt% and the obtained material was colorless. Isopropyl alcohol was detected after the formation of the hybrid films, confirming the reaction of PVA with the isopropoxy group in the titanium phosphonate cluster. The application of titanium phosphonate clusters for the preparation of hybrid materials is expected to be useful for the development of new organic–inorganic hybrid materials, including various reactive positions in a molecule.

Synthesis of TiOPPh. TiOPPh, Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf.
References
Cordes, D. B., Lickiss, P. D. & Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 110, 2081–2173 (2010).
Bradley, D. C., Mehrotra, R. C., Rothwell, I. P. & Singh, A. Alkoxo and Aryloxo Derivatives of Metals (Academic Press, San Diego, CA, USA, 2001).
Schubert, U. Organofunctional metal oxide clusters as building blocks for inorganic-organic hybrid materials. J. Sol-Gel Sci. Technol. 31, 19–24 (2004).
Chujo, Y. & Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn 88, 633–643 (2015).
Rozes, L., Steunou, N., Fornasieri, G. & Sanchez, C. Titanium-oxo clusters, versatile nanobuilding blocks for the design of advanced hybrid materials. Monatsh. Chem. 137, 501–528 (2006).
Gross, S. Oxocluster-reinforced organic–inorganic hybrid materials: effect of transition metal oxoclusters on structural and functional properties. J. Mater. Chem. 21, 15853–15861 (2011).
Schubert, U. Cluster-based inorganic–organic hybrid materials. Chem. Soc. Rev. 40, 575–582 (2011).
Kickelbick, G. Hybrid Materials (Wiley VCH, Weinheim, Germany, 2006).
Tanaka, K., Yamane, H., Mitamura, K., Watase, S., Matsukawa, K. & Chujo, Y. Transformation of sulfur to organic–inorganic hybrids employed by networks and their application for the modulation of refractive indices. J. Polym. Sci. Part A: Polym. Chem. 52, 2588–2595 (2014).
Guerrero, G., Mutin, P. H. & Vioux, A. Mixed nonhydrolytic/hydrolytic sol−gel routes to novel metal oxide/phosphonate hybrids. Chem. Mater. 12, 1268–1272 (2000).
Guerrero, G., Mehring, M., Mutin, P. H., Dahan, F. & Vioux, A. Syntheses and single-crystal structures of novel soluble phosphonate- and phosphinato-bridged titanium oxo alkoxides. J. Chem. Soc., Dalton Trans. 1537–1538 (1999).
Mehring, M., Guerrero, G., Dahan, F., Mutin, P. H. & Vioux, A. Syntheses, characterizations, and single-crystal X-ray structures of soluble titanium alkoxide phosphonates. Inorg. Chem. 39, 3325–3332 (2000).
Czakler, M., Artner, C. & Schubert, U. Influence of the phosphonate ligand on the structure of phosphonate-substituted titanium oxo clusters. Eur. J. Inorg. Chem. 2013, 5790–5796 (2013).
Czakler, M., Artner, C. & Schubert, U. Acetic acid mediated synthesis of phosphonate-substituted titanium oxo clusters. Eur. J. Inorg. Chem. 2014, 2038–2045 (2014).
Czakler, M., Artner, C. & Schubert, U. Titanium oxo/alkoxo clusters with both phosphonate and methacrylate ligands. Monatsh. Chem. 146, 1249–1256 (2015).
Kalita, L., Kalita, A. C. & Murugavel, R. Organotitanium phosphates with free P–OH groups: synthesis, spectroscopy and solid state structures. J. Organomet. Chem. 751, 555–562 (2014).
Sheldrick, G. M. SADABS, Program for Siemens Area Detector Absorption Correction (University of Göttingen, Germany, 1996).
Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
Armarego, W. L. F. & Chai, C. Purification of Laboratory Chemicals 7th edn (Elsevier, Oxford, UK, 2012).
Otsuka, T. & Chujo, Y. Poly(methyl methacrylate) (PMMA)-based hybrid materials with reactive zirconium oxide nanocrystals. Polym. J. 42, 58–65 (2010).
Yeh, J.-M., Weng, C.-J., Huang, K.-Y., Huang, H.-Y., Yu, Y.-H. & Yin, C.-H. Thermal and optical properties of PMMA-titania hybrid materials prepared by sol-gel approach with HEMA as coupling agent. J. Appl. Polym. Sci. 94, 400–405 (2004).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘New Polymeric Materials Based on Element-Blocks (No. 2401)’ (JSPS KAKENHI Grant Number JP24102008). This work was supported by JSPS KAKENHI Grant Number JP16K17951.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hayami, R., Wada, K., Sagawa, T. et al. Preparation and properties of organic–inorganic hybrid polymer films using [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(PhPO3)3]·thf. Polym J 49, 223–228 (2017). https://doi.org/10.1038/pj.2016.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2016.108
This article is cited by
-
A review of phosphorus(V)-substituted titanium-oxo clusters
Journal of Sol-Gel Science and Technology (2021)
-
Preparation and properties of methyl- and cyclohexylsilsesquioxane oligomers as organic–inorganic fillers
Journal of Sol-Gel Science and Technology (2020)
-
Soluble ethane-bridged silsesquioxane polymer by hydrolysis–condensation of bis(trimethoxysilyl)ethane: characterization and mixing in organic polymers
Journal of Polymer Research (2020)
-
Organic–inorganic hybrids based on poly(bisphenol A-co-epichlorohydrin) containing titanium phosphonate clusters
Polymer Journal (2019)
-
Properties and surface morphologies of organic–inorganic hybrid thin films containing titanium phosphonate clusters
Polymer Journal (2018)