Abstract
The gene encoding phosphatidylinositol 3-kinase catalytic subunit α-isoform (PIK3CA, p110α) is frequently activated by mutation in human cancers. Based on detection in some breast cancer precursors, PIK3CA mutations have been proposed to have a role in tumor initiation. To investigate this hypothesis, we generated a novel mouse model with a Cre-recombinase regulated allele of p110α (myristoylated-p110α, myr-p110α) along with p53fl/fl deletion and KrasG12D also regulated by Cre-recombinase. After instillation of adenovirus-expressing Cre-recombinase into mammary ducts, we found that myr-p110α accelerated breast tumor initiation in a copy number-dependent manner. Breast tumors induced by p53fl/fl;KrasG12D with no or one copy of myr-p110α had predominantly sarcomatoid features, whereas two copies of myr-p110α resulted in tumors with a carcinoma phenotype. This novel model provides experimental support for importance of active p110α in breast tumor initiation, and shows that the amount of PI3K activity can affect the rate of tumor initiation and modify the histological phenotype of breast cancer.
Similar content being viewed by others
Introduction
Receptor tyrosine kinase-phosphatidylinositol 3-kinase (PI3K) signaling is a central integrator of metabolism, cell growth and cell survival, and deregulated PI3K signaling increases tumorigenicity.1, 2 Cancer-associated mutations occur in several components of the pathway including activating mutations of both the PI3K catalytic subunit α-isoform gene (PIK3CA, p110α)3 and the downstream signaling molecule AKT1,4 as well as deletion of the negative pathway regulator, PTEN.5 These alterations all result in increased activity of the PI3K signaling pathway. The significance of p110α in cancer is demonstrated by the high frequency of activating mutations in many common human cancers that increase the catalytic activity of PI3K.3, 6
High-throughput RNA sequencing and tumor resequencing have revealed that hyperactivating mutations in the PI3K signaling pathway occur in a substantial percentage of breast cancers.7 The PIK3CA gene itself, encoding the p110α catalytic subunit, is the most frequently mutated gene in breast cancer,8, 9 with mutations in 25–40% of all breast cancers.10, 11 However, the prognostic implications of p110α mutations remain unclear. Although the association of PI3K mutations with poor prognosis has been reported,12, 13 others have described a correlation with improved outcome.14, 15
Whole-exome analysis of finely dissected and matched mammary tumors has shown that alterations in the p110α gene are detected at the same frequencies in ductal carcinoma in situ (DCIS), DCIS adjacent to invasive carcinoma and invasive carcinoma.16 In addition, the p110α sequences are often found to be identical in invasive and in situ areas of the same breast tumor.16 This pattern of mutation suggests that p110α mutations are a relatively early event in breast cancer development. In light of these findings, mutational activation of the PI3K signaling pathway has been proposed to have a role in breast tumor initiation.
Multiple mouse models of the PI3K pathway-driven cancer have recently been developed to investigate the impact of PI3K mutation or combination of other related mutations on breast cancer development under the control of MMTV LTR or WAP promoter.17, 18, 19, 20, 21 However, the role of PI3K activation in tumor initiation, progression and phenotype is not well understood. In the present study, the PI3K catalytic subunit p110α was myristoylated to force localization to the cell membrane, which is sufficient to release its kinase activity from normal regulation, and to thus activate the PI3K signaling pathway. The expression of myristoylated-p110α was regulated by Cre-recombinase expressed from Ad-Cre virus, which allowed for spatial and temporal experimental control. To ascertain the impact of p110α activating mutations in tumor initiation and progression, we combined myr-p110α with frequently co-occurring mutations of two genes, p53 and Kras, to generate novel models of breast, lung and potentially other cancers whose induction is controlled by the researcher.
Results
In vivo expression of myr-p110α and homozygous p53 deletion in milk duct causes slowly developing mammary tumors
To elucidate the role of activating mutations of p110α in tumorigenesis, we engineered a mouse with conditional myristoylated-p110α (myr-p110α) ubiquitously expressed from the Rosa26 locus (Supplementary Figure 1).22 Constitutive expression of myr-p110α during development was embryonic lethal.22 To have temporal and spatial control over tumorigenic genetic changes in breast cancer, we utilized in vivo administration of adenovirus-expressing Cre-recombinase (Ad-Cre) into the mammary duct of virgin females.23
As activated myr-p110α by itself had minimal transforming activity (Supplementary Figure 2) and the p53 gene is the second most frequently mutated gene in breast cancer,8, 24 we assessed the effect of myr-p110α and homozygous p53 deletion on breast tumor development. In line with its tumorigenic potential in mouse embryonic fibroblasts (MEFs; Supplementary Figure 2) and previous studies showing the cooperation between Pik3caH1047R activating mutation and p53 knockout in vivo,17, 20 mice expressing one copy of myr-p110α and homozygous for p53 deletion (p53fl/fl;myr-p110αwt/fl) developed mammary tumors with 100% penetrance but with a long latency (mean tumor onset 259.6±10.6 days; Figure 1a). Histological analysis of the tumors revealed predominantly poorly differentiated or dedifferentiated malignant neoplasms with sarcomatoid features as well as small areas of high-grade carcinoma. The sarcomatoid neoplasms were positive for estrogen receptor α (ERα), focal progesterone receptor (PR) and vimentin (mesenchymal marker); they were negative for cytokeratin 5 (CK5, basal epithelial marker), cytokeratin 14 (CK14, myoepithelial marker), cytokeratin 8 (CK8, luminal epithelial marker) and human epidermal growth factor receptor 2 (Her2/neu; Figure 1b). In contrast, mice harboring either myr-p110αwt/fl or homozygous p53 deletion only, failed to form mammary tumors during 12 months of observation.
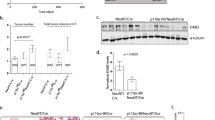
In vivo expression of myr-p110α and homozygous p53 deletion in milk duct activates PI3K signaling and develops mammary tumors. (a) Kaplan–Meier survival curve demonstrating breast tumor onset defined as the first palpation-mediated recognition of tumors (n=10 per group). Log-rank (Mantel–Cox) test was used for statistical analysis. ***P<0.001. (b) Hematoxylin and eosin (H&E) staining and immunohistochemistry of mammary tumors from p53fl/fl;myr-p110αwt/fl females stained with antibodies against following markers: ERα (estrogen receptor α), PR (progesterone receptor), vimentin (mesenchymal marker), CK5 (basal marker), CK14 (myoepithelial marker), CK8 (luminal marker) and Her2/neu. Representative pictures of H&E staining and immunostaining were taken with × 200 magnification. Scale bar 100 μm indicates scale for all images. The poorly differentiated neoplasm is immunoreactive with ERα and vimentin, and negative for the remaining antigens. A normal duct serves as an internal control (arrow in H&E). (c) Western blot analysis of p110α, p-AKT, AKT and GFP with normal mammary tissue and mammary tumors from p53fl/fl;myr-p110αwt/fl mice. (d) The graphs of western blot signals of p110α, p-AKT and p-AKT/AKT ratio quantified and normalized with respect to β-actin. (e) Western blot analysis of p85α, p-PTEN and PTEN with wild-type mammary tissue and p53fl/fl;myr-p110αwt/fl mammary tumors. (f) The graphs of western blot signals of p85α, p-PTEN, PTEN and p-PTEN/PTEN ratio quantified and normalized with respect to β-actin. For d and f, mean fold increase compared with mammary tissue lysates from a non-Cre harboring age-matched female was calculated after normalization. Error bars are the mean±s.e.m. Two-tailed unpaired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01 and ***P<0.001. Data in c–f are representative of four independent experiments with biological and technical replicates.
Mammary tumors expressing p53fl/fl;myr-p110αwt/fl activate PI3K signaling
Increased PI3K signaling activates AKT, a key downstream effector of PI3K signaling pathway.25 To test for PI3K signaling activation, mammary tumors were analyzed for levels of total p110α, total AKT, the active phosphorylated form of AKT (p-AKT), PI3K regulatory subunit p85α, PI3K negative regulator PTEN and the less active phosphorylated form of PTEN with reduced lipid phosphatase activity (p-PTEN).26, 27 As compared with normal mammary tissue, mammary tumors from p53fl/fl;myr-p110αwt/fl females had elevated levels of total p110α (32-fold), p-AKT (37-fold) and p-AKT/AKT ratio (34-fold; Figures 1c and d). Expression of myr-p110α was confirmed by the detection of GFP in mammary tumors (Figure 1c). The p53fl/fl;myr-p110αwt/fl mammary tumors also displayed significantly increased total protein levels of p85α (1.9-fold), PTEN (1.3-fold) and p-PTEN (1.7-fold; Figures 1e and f), suggesting naturally occurring negative feedback mechanisms to inhibit abnormal PI3K activity driven by myr-p110α. Elevated levels of p-AKT were associated with the increased PI3K activity despite increased levels of PI3K regulators, p85α and PTEN, demonstrating ineffective regulation by increased p85α and PTEN.
Addition of myr-p110α into p53fl/fl;KrasG12D double mutants accelerates mammary tumor initiation but not tumor growth rate
It has been proposed that PIK3CA has a role in tumor initiation, as its mutations are detected in precursors of breast cancer.28 To investigate the role of myr-p110α in tumor initiation and progression, we exploited the Cre-inducible oncogenic mouse model p53fl/fl;KrasG12D, which harbors a homozygous p53 deletion and a KrasG12D activating missense allele that rapidly grows tumors.29 Based on the published breast cancer genomics data, KrasG12D mutation tends to co-occur with p53 mutations (Supplementary Figures 3A and 3B). To assess tumor initiation and development in p53fl/fl;KrasG12D mice in the absence or presence of myr-p110α, mice harboring p53fl/fl;KrasG12D or p53fl/fl;KrasG12D;myr-p110αwt/fl were generated. Ad-Cre virus was administered into the milk duct of virgin females and mice were monitored for initial tumor formation and growth rate. Both p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice developed mammary tumors with 100% penetrance. Notably, although p53fl/fl;KrasG12D mice began to form palpable mammary tumors (tumor size ranges 12–15 mm2) with a mean tumor onset of 54.8±0.7 days, p53fl/fl;KrasG12D;myr-p110αwt/fl mice started to form palpable mammary tumors with a mean tumor onset of 28.7±0.4 days (Figure 2a), indicating roughly two-fold acceleration of tumor initiation due to expression of myr-p110α.
Addition of myr-p110α into p53fl/fl;KrasG12D double mutants increases PI3K signaling and accelerates mammary tumor initiation with no impact on tumor growth rate. (a) Kaplan–Meier survival curve demonstrating breast tumor onset in the two strains. Log-rank (Mantel–Cox) test was used for statistical analysis. ***P<0.001. (b) Kaplan–Meier survival curve demonstrating mammary tumor progression in each strain. The period of tumor growth was defined as the number of days between when a mammary tumor was first observed in the size range of 12–15 mm2 and when tumor surface area reached the end point of 100 mm2. Log-rank (Mantel–Cox) test was used for statistical analysis. P>0.05, non-significant. (c) Western blot analysis of p110α, p-AKT, AKT and GFP with wild-type (WT) mammary tissue and mammary tumors from p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice. (d) The graphs of western blot signals of p110α, p-AKT, p-AKT/AKT ratio and AKT quantified and normalized with respect to β-actin. (e) Western blot analysis of p85α, p-PTEN and PTEN with WT mammary tissue and mammary tumors from p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice. (f) The graphs of western blot signals of p85α, p-PTEN, PTEN and p-PTEN/PTEN ratio quantified and normalized with respect to β-actin. Mean fold increase compared with mammary tissue lysates from a non-Cre harboring age-matched female was calculated after normalization. Error bars are the mean±s.e.m. Two-tailed unpaired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01 and ***P<0.001. Data in c–f are representative of four independent experiments with biological and technical replicates.
To examine the difference in tumor progression, the period of tumor growth was defined as the number of days between when a mammary tumor was first observed and when the animal reached end point, and depicted in the Kaplan–Meier survival curve. Although initiation was markedly accelerated, once the tumor was established, there was no difference in tumor growth rate between p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice. Mammary tumors from p53fl/fl;KrasG12D mice reached end point with a mean of 18.7±0.7 days, and p53fl/fl;KrasG12D;myr-p110αwt/fl mammary tumors reached end point with a mean of 18.4±0.5 days (Figure 2b).
Mammary tumors from p53fl/fl; KrasG12D ;myr-p110αwt/fl have higher p-AKT levels than those from p53fl/fl ;KrasG12D
To biochemically characterize mammary tumors from p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl, we tested the PI3K pathway activation status of the mammary tumors by analyzing the levels of p110α, AKT, p-AKT, p85α, PTEN and p-PTEN.
Mammary tumors from p53fl/fl;KrasG12D females had significantly elevated levels of total p110α (4.5-fold), p-AKT (11-fold) and p-AKT/AKT ratio (10-fold) as shown in Figures 2c and d, in comparison with normal mammary tissue. p53fl/fl;KrasG12D;myr-p110αwt/fl mammary tumors with one copy of myr-p110α had further elevated expression levels of total p110α (39-fold), and this resulted in increased p-AKT levels by 48-fold and increased p-AKT/AKT ratio (52-fold; Figures 2c and d). Expression of myr-p110α via Ad-Cre virus injection was confirmed by GFP expression in p53fl/fl;KrasG12D;myr-p110αwt/fl mammary tumors (Figure 2c).
Mammary tumors from p53fl/fl;KrasG12D;myr-p110αwt/fl mice had elevated accumulation of regulatory subunit p85α by two-fold as compared with normal mammary tissue, whereas p53fl/fl;KrasG12D mammary tumors displayed significantly decreased p85α level (0.6-fold) as shown in Figures 2e and f. The PTEN levels had a modest but statistically significant 0.6-fold decrease in p53fl/fl;KrasG12D mammary tumors compared with normal mammary tissue (Figures 2e and f). The ratios of p-PTEN/PTEN were slightly but significantly increased in p53fl/fl;KrasG12D;myr-p110αwt/fl (1.3-fold) mammary tumors (Figure 3f), indicating overall increase of less active p-PTEN.
Two copies of myr-p110α further increase PI3K activity and accelerate mammary tumor initiation and growth rate. (a) Kaplan–Meier survival curve demonstrating the breast tumor onset in three strains: p53fl/fl;KrasG12D, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl. (b) Kaplan–Meier survival curve demonstrating the breast tumor onset in three strains: p53fl/fl, p53fl/fl;myr-p110αwt/fl and p53fl/fl;myr-p110αfl/fl. (c) Western blot analysis of p110α, p-AKT and AKT with mammary tumors from p53fl/fl;myr-p110αwt/fl, p53fl/fl;myr-p110αfl/fl, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl mice. (d) The graphs of western blot signals of p110α, p-AKT and p-AKT/AKT ratio quantified and normalized with respect to β-actin. Mean fold increase compared with p53fl/fl;myr-p110αwt/fl mammary tumors was calculated after normalization. Error bars are the mean±s.e.m. Two-tailed unpaired Student’s t-test was used for statistical analysis. *P<0.05, **P<0.01 and ***P<0.001. Data in c and d are representative of three independent experiments with biological and technical replicates. (e) Kaplan–Meier survival curve demonstrating mammary tumor progression in three strains: p53fl/fl;KrasG12D, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl. The period of tumor growth was defined as the number of days between when a mammary tumor was first observed in the size range of 12–15 mm2 and when tumor size reached 100 mm2. For a, b and e, Log-rank (Mantel–Cox) test was used for statistical analysis with **P<0.01 and ***P<0.001. To determine whether copy number increase in myr-p110α would have further impact on tumor initiation and progression in breast cancer models, the data generated from p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl females shown in the Figures 2a and b, and the data generated from p53fl/fl and p53fl/fl;myr-p110αwt/fl females shown in the Figure 1a, were replicated for comparison.
Two copies of myr-p110α further accelerate mammary tumor initiation and promote tumor progression
To determine how doubling the copy number of myr-p110α would impact tumorigenesis in this breast cancer model, we generated homozygous mice with two copies of myr-p110α transgene along with p53fl/fl;KrasG12D mutants (p53fl/fl;KrasG12D;myr-p110αfl/fl). After Ad-Cre virus administration into the milk duct of virgin females, the time of tumor onset and tumor growth rate of p53fl/fl;KrasG12D;myr-p110αfl/fl mammary tumors were compared with those of p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mammary tumors to assess the effect of homozygous myr-p110α alleles.
Intraductal delivery of Ad-Cre virus into p53fl/fl;KrasG12D;myr-p110αfl/fl mice induced the development of mammary tumors with 100% penetrance and a mean tumor onset of 15.7±0.6 days (Figure 3a). This tumor initiation was much faster than p53fl/fl;KrasG12D;myr-p110αwt/fl mice (one copy of myr-p110α, 28.7±0.4 days) and p53fl/fl;KrasG12D mice (zero copy of myr-p110α, 54.8±0.7 days). In accordance with this result, addition of two copies of myr-p110α into homozygous p53 deletion (p53fl/fl;myr-p110αfl/fl) also facilitated mammary tumor initiation compared with p53fl/fl;myr-p110αwt/fl (135.1±6.2 days vs 259.6±10.6 days; Figure 3b).
To check whether there would be a correlation between the extent of PI3K signaling activation and the rate of tumor initiation, we evaluated the PI3K signaling activation status by comparing the levels of p-AKT between p53fl/fl;myr-p110αwt/fl, p53fl/fl;myr-p110αfl/fl, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl mammary tumors. Two copies of myr-p110α significantly elevated levels of total p110α (1.9-fold) and p-AKT (2.1-fold), as compared with one copy of myr-p110α (Figures 3c and d). We also tested the extent of PI3K signaling activation in mammary tumors harboring zero, one or two copies of myr-p110α transgene combined with either p53fl/fl or p53fl/fl;KrasG12D mutants. The levels of PI3K signaling activation were significantly elevated in a myr-p110α copy number-dependent manner (Supplementary Figure 4). These data demonstrate that a single copy of myr-p110α does not saturate the PI3K signaling pathway with regard to p-AKT levels and two copies of myr-p110α further activate PI3K signaling to a greater degree than that induced by a single copy of myr-p110α in mammary tumors, resulting in the increased rate of tumor initiation.
As the rate of tumor initiation was further influenced by the addition of KrasG12D mutation into p53fl/fl;myr-p110α, which has been shown to signal primarily through the PI3K/AKT pathway,30 we assessed the levels of PI3K signaling activation in p53fl/fl;myr-p110α mammary tumors with or without KrasG12D mutation. Mammary tumors from p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl had significantly increased levels of total p110α (1.3-fold) and p-AKT levels (1.3-fold), as compared with p53fl/fl;myr-p110αwt/fl and p53fl/fl;myr-p110αfl/fl mammary tumors as shown in Figures 3c and d. These findings show that PI3K signaling can be further activated by cooperation of myr-p110α with KrasG12D mutation, and the rate of tumor initiation is strongly influenced by the level of PI3K activity.
Interestingly, the addition of two copies of myr-p110α also modestly but significantly accelerated tumor progression, as revealed by the survival curve (Figure 3e). Mammary tumors from p53fl/fl;KrasG12D;myr-p110αfl/fl mice reached end point with a mean of 15.4±0.8 days, faster than p53fl/fl;KrasG12D;myr-p110αwt/fl mice (18.4±0.5 days) and p53fl/fl;KrasG12D mice (18.7±0.7 days).
Addition of myr-p110α into p53fl/fl ;KrasG12D double mutants modifies histologic phenotype
Given the inherent heterogeneity of breast cancers, mammary tumors from the transgenic mice were characterized histologically (Figure 4). Mammary tumors from p53fl/fl;KrasG12D mice were mostly poorly differentiated malignant neoplasms with sarcomatoid features. Mammary tumors from p53fl/fl;KrasG12D;myr-p110αwt/fl also had a predominantly sarcomatoid histologic phenotype similar to p53fl/fl;KrasG12D mammary tumors, but ~10–15% of tumor area was better differentiated with overt epithelial components including DCIS, papillary carcinoma and invasive ductal carcinoma.
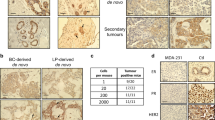
Addition of myr-p110α allele into p53fl/fl;KrasG12D double mutants modifies tumor histology in a copy number-dependent manner. (a) Representative hematoxylin and eosin (H&E) sections of mammary tumors from p53fl/fl;KrasG12D, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl mice showing increasing epithelial differentiation with addition of myr-p110α allele. Scale bar represents 0.5 mm and indicates scale for all images. (b) Comparison of histologic and immunohistochemical (CK8, ERα, PR and vimentin) phenotypes between p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αfl/fl tumors. Representative H&E sections show solid sheets and fascicles of pleomorphic spindle cells in p53fl/fl;KrasG12D tumor compared to the epithelial nests and glands present in p53fl/fl;KrasG12D;myr-p110αfl/fl tumor. Both tumors are immunoreactive with CK8 and ERα; the p53fl/fl;KrasG12D tumor is PR negative and vimentin positive, whereas the p53fl/fl;KrasG12D;myr-p110αfl/fl tumor is PR positive and vimentin negative. Scale bar represents 100 μm. Magnification is × 40 (a), × 400 (ERα staining of the p53fl/fl;KrasG12D tumor in b) or × 200 (all remaining images in b).
Histological analysis of mammary tumors from p53fl/fl;KrasG12D;myr-p110αfl/fl mice demonstrated an increase in tumors with a carcinoma phenotype including both DCIS and invasive ductal carcinoma (Figure 4a). It was also noted that there was an increase in benign epithelial proliferations including adenosis and papillomas. Some of the tumors had an admixed spindle cell component. As the copy number of myr-p110α doubled, the majority of p53fl/fl;myr-p110αfl/flmammary tumors also developed a carcinoma phenotype, whereas p53fl/fl;myr-p110αwt/fl mammary tumors developed dedifferentiated mixed tumors with sarcomatoid features and only focal areas of carcinoma (Supplementary Figure 5). These findings show that increased PI3K activity mediated by two copies of myr-p110α, when combined with either p53fl/fl or p53fl/fl;KrasG12D, have the potential to skew mammary tumors from a sarcomatoid phenotype to a carcinoma phenotype more common in humans.
As part of the pathological characterization, tumors were stained for the following markers: CK5, CK14, CK8, ERα, PR, Her2/neu and vimentin. The majority of mammary tumors (invasive and in situ carcinoma, and benign proliferations) from p53fl/fl;KrasG12D;myr-p110αfl/fl mice displayed strong CK8, ERα and PR immunostaining (Figure 4b). They were negative for vimentin (Figure 4b), whereas CK5 and CK14 immunostains confirmed the presence of myoepithlelial cells within DCIS and benign epithelial proliferations (data not shown). The sarcomatoid tumors from p53fl/fl;KrasG12D mice were strongly positive for vimentin and showed variable expression of CK8, ERα and PR (Figure 4b). They were negative for CK5 and CK14. Tumors from p53fl/fl;KrasG12D;myr-p110αwt/fl mice displayed greater immunostaining for CK8 compared to p53fl/fl;KrasG12D mice (data not shown). None of the tumors from the three groups demonstrated positive (3+) Her2/neu immunostaining.
Addition of myr-p110α into p53fl/fl;KrasG12D increases metastatic potential
To assay the ability of breast tumor cells to establish tumors outside the breast, we derived cells from p53fl/fl;KrasG12D, p53fl/fl;KrasG12D;myr-p110αwt/fl and p53fl/fl;KrasG12D;myr-p110αfl/fl mammary tumors, introduced them into the circulation by tail vein injection into syngeneic mice, and monitored for the formation of breast tumors in the lungs, as this method is considered to be a relevant model for breast cancer lung metastasis.31 17 days after injection, mice injected with the p53fl/fl;KrasG12D;myr-p110αfl/fl breast tumor cells produced significantly more identifiable tumors in the lungs (mean 318.6±7.4) compared with mice injected either with p53fl/fl;KrasG12D breast tumor cells (mean 6.3±2.6) or p53fl/fl;KrasG12D;myr-p110αwt/fl breast tumor cells (mean 206.1±10.0; Figure 5). This suggests that the myr-p110α promotes growth of metastatic tumors in a copy number-dependent manner.
Combination of myr-p110α with p53fl/fl;KrasG12D increases metastatic potential in a myr-p110α copy number-dependent manner. The graph showing the number of tumor lesions found in the lung surface 17 days after intravenous injection of breast tumor cells with different genotypes into the tail vein of syngeneic hosts. Error bars are the mean±s.e.m. Two-tailed unpaired Student’s t-test was used for statistical analysis. ***P<0.001. In parallel, Mann–Whitney test (Wilcoxon rank-sum test) was used to reject the null hypothesis that there is no difference between groups (***Mann–Whitney U-value=0.0009). Data are representative of three independent experiments using n=8 per group with biological and technical replicates. Six- to eight-week-old syngeneic host mice were randomly assigned to generate similar numbers of subjects among groups.
Addition of myr-p110α allele into p53fl/fl;KrasG12D double mutants accelerates tumor initiation in lung and decreases survival
One intentional aspect of the generation of this Cre-inducible mouse model and the utilization of Ad-cre virus is the ability to genetically modify different anatomic locations and potentially cause different tumor types. For the generation of lung cancer, we utilized injection of Ad-Cre virus into the trachea without surgery.
Both p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice developed lung tumors with 100% penetrance. Histological analysis of lung tumors 52 days after intratracheal injection of Ad-Cre virus (when the first mice had symptoms of lung tumor growth) revealed high-grade adenocarcinomas that were largely disseminated throughout the lungs of p53fl/fl;KrasG12D;myr-p110αwt/fl mice, whereas p53fl/fl;KrasG12D mice developed only focal, small areas of adenocarcinoma (Figure 6a). Accordingly, lungs from p53fl/fl;KrasG12D;myr-p110αwt/fl mice were enlarged and weighed two to three times more than lungs from either p53fl/fl;KrasG12D mice or non-tumor-bearing mice, defining more tumor burdens (Figure 6b). These findings indicate that the addition of myr-p110α promotes lung tumor development. In contrast with breast cancer, the addition of one copy of myr-p110α into p53fl/fl;KrasG12D did not change histology of the lung tumors. To further define the characteristics of the lung tumor development, mice were allowed to reach end point. The mean survival of p53fl/fl;KrasG12D;myr-p110αwt/fl mice was 55±1.4 days, whereas the mean survival of p53fl/fl;KrasG12D mice was 112±1.6 days, (Figure 6c). This finding was similar to the breast tumor experiments, in which survival time was cut in half by addition of one copy of myr-p110α. Overall, our observations demonstrate that the addition of constitutively active myr-p110α allele causes early tumor initiation in the lung, leading to early death related to lung tumors.
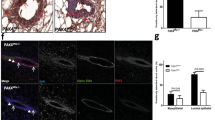
Addition of one copy of myr-p110α into p53fl/fl;KrasG12D double mutants accelerates tumor development in lung. (a) Hematoxylin and eosin (H&E) staining of lungs dissected from p53fl/fl;KrasG12D and p53fl/fl;KrasG12D;myr-p110αwt/fl mice 52 days post Ad-Cre virus injection. Pictures of H&E staining were taken with × 40 magnification and are representative. Scale bar represents 1 mm and indicates scale for all images. (b) Bar graphs describing the weight of lung divided by body weight, 52 days after Ad-Cre virus injection. Error bars are the mean±s.e.m. Two-tailed unpaired Student’s t-test was used for statistical analysis. ***P<0.001. (c) Kaplan–Meier survival curve scoring mice showing symptoms from lung tumor growth defined as any symptoms associated with illness. Log-rank (Mantel–Cox) test was used for statistical analysis. ***P<0.001. Data in b and c are representative of three independent experiments using n=5–6 per group.
Discussion
Impact of constitutively active PI3K on tumor initiation and progression
It has recently been shown that cell signaling in many breast cancers is associated with an activated PI3K/AKT pathway.32 Notably, by evaluating differences in the p-AKT levels between mammary tumors harboring zero, one or two copies of myr-p110α allele combined with either p53fl/fl or p53fl/fl;KrasG12D mutants, our results proved that the levels of PI3K signaling activation are significantly increased and strongly correlated with the increased rate of tumor initiation in a myr-p110α copy number-dependent manner. These data demonstrated that higher levels of PI3K activity induced by myr-p110α addition have profound effects on tumor initiation. In addition, our results showed that the biological activity of PI3K signaling can be further activated by cooperation of myr-p110α with KrasG12D mutation. Our data from the use of breast tumor cells also determined that the addition of myr-p110α allele into p53fl/fl;KrasG12D mutants facilitates engraftment in the lungs, as an assay of metastatic potential, in a copy number-dependent manner. Overall, our findings provide experimental evidence that PI3K activating mutations associated with increased PI3K signaling activity accelerate the rate of tumor initiation and increase potential for metastasis.
Although p53fl/fl;KrasG12D;myr-p110αwt/fl mice did not have increased growth rates of breast tumors as compared with p53fl/fl;KrasG12D mice once tumors had developed, there was a modest but significant increase in the growth rate of tumors following initiation in p53fl/fl;KrasG12D mice with two copies of myr-p110α as compared with p53fl/fl;KrasG12D mice and p53fl/fl;KrasG12Dmice with one copy of myr-p110α. One potential interpretation for these results is that sufficiently high levels of PI3K/AKT signaling activation also contribute to tumor growth rate.
Impact of dosage of constitutively active PI3K on breast tumor phenotype
In our study, the addition of myr-p110α allele into p53fl/fl;KrasG12D or p53fl/fl that resulted in significantly increased PI3K/AKT signaling activation generated a carcinoma histologic phenotype in a myr-p110α copy number-dependent manner. The resulting tumors induced by the addition of myr-p110α allele resembled those that are commonly seen in the majority of human breast cancer. This was evident by the morphology of the invasive carcinoma, as well as by the increased presence of DCIS and benign epithelial proliferations. Thus, these findings suggest that levels of PI3K signaling might have a distinct impact on developing carcinoma phenotypes in humans. As the histological phenotype of tumors with two copies of myr-p110α was quite different than tumors with one copy, the increased growth rate induced by two copies could be attributed to the difference in tumor type generated by one versus two copies. One limitation of our study is that we did not define the relation between the spatial expression levels of myr-p110α or resulting p-AKT levels and histological features within the specific tumor areas. Additional studies are warranted to further evaluate this association between PI3K activity and tumor differentiation.
Value of triple mutant transgenic mice for human cancer model
The value of genetically modified mouse models for cancer studies lies in the uniformity of the mouse background, the speed of cancer development enabling practical studies, and the similarity to human disease both genetically and histopathologically. The inherent heterogeneity of human breast cancer with variant histopathologic features even within a single tumor33, 34 creates an enormous challenge for understanding exact mechanisms of disease pathology. Our goal was to develop a breast cancer model that recapitulates the most common histopathological breast tumors, is temporally inducible and spatially controlled by the experimenter, and initiates and develops rapidly to facilitate practical experimentation.
Current mouse breast tumors driven by PI3KCA activating mutations mimic histopathologic phenotypes observed in human breast cancers;17, 18, 19, 20, 35 however, these single genetic mutation mouse models can take a year to develop breast tumors. Our mouse model that combines one or two copies of myr-p110α with p53fl/fl;KrasG12D can resemble human breast cancer subtypes, as well as develop tumors early and grow quickly, leading to a shortened experimental timeline from tumor initiation to end stage.
Spontaneous additional mutations have been demonstrated to accumulate along with epigenetic events resulting in an increased level of genetic complexity in human cancer.36 The mouse model reported here is unique in that it harbors a PI3KCA (p110α) oncogene, which is frequently mutated in many human cancers,3, 6 along with the tumor suppressor gene p53 deletion and a well-known oncogene, KrasG12D. These mutations are repeatedly altered in human cancers, so they represent relevant genetic alterations that can be used in developing models to closely mimic the pathological and biochemical features of cancers. In addition, this new mouse model is also able to rapidly generate cancers in other tissues by the application of Cre-recombinase with adenovirus as demonstrated by lung cancer generation, and it will be of scientific value to utilize this model for studying other tumor types. As the mutations require Cre-recombinase for manifestation, the triple mutation mouse lines can be maintained easily since breeding is not affected.
In summary, this novel triple mutation model system (1) is an autochthonous genetic model of human cancer, (2) provides controlled inducible expression of genetic changes spatially and temporally, (3) induces tumor subtypes matched histologically to human breast tumors, and (4) develops tumors very rapidly. The characteristics outlined should make this new genetically modified mouse model of significant value for understanding the molecular mechanisms underlying development of various tumors.
Materials and methods
Generation of experimental transgenic mice
The transgenic Cre-inducible myristoylated-p110α mice on C57BL/6 backgrounds were generated22 and intercrossed with Cre-inducible mice carrying either only p53 deletion homozygote (p53fl/fl, which deletes exon 2 to exon 10 leading to null alleles of p53 upon Cre-mediated excision)37 or p53fl/fl and KrasG12D mutations29 (p53fl/fl;KrasG12D, intercrossed) on C57BL/6 backgrounds. Conditional mutant mice with various genotypes were obtained and genotyped by PCR. The animal study protocol was reviewed and approved by the institutional animal care and use committee of Geisel School of Medicine at Dartmouth.
In vivo intraductal delivery of Adeno-Cre virus into mammary duct
Six- to eight-week-old virgin females were anesthetized by isofluorane/oxygen inhalation. The calcium phosphate precipitate containing 2.5 × 107 plaque-forming unit of Ad-Cre virus was injected into the teat canal of fourth inguinal mammary duct using a Hamilton syringe with 33-G needle.23 Mice were monitored daily for a palpable/visible tumor formation. Tumor surface area was calculated by the formula: tumor size (mm2)=length (mm) × width (mm), after caliper measurements.
Hematoxylin and eosin stain
Paraffin-embedded tissues after fixation with 10% buffered formalin (15740-01, Electron Microscopy Sciences, Hatfield, PA, USA) were sectioned at 6 μm. Deparaffinized sections were stained with hematoxylin and eosin following the manufacturer’s protocol and mounted with Permount (SP15-100, Fisher Scientific, Grand Island, NY, USA). Images were obtained on an Olympus BX5.1 or BX4 microscope (Olympus, Waltham, MA, USA), and captured using Image Pro software (Media Cybernetics, Rockville, MD, USA).
Immunohistochemistry
Tumors were paraffin-embedded after fixation in 10% buffered formalin and sectioned at 6 μm. Sections were immunostained using standard manufacturer’s protocols at the Dartmouth-Hitchcock Medical Center Laboratory for Clinical Genomics and Advanced Technology with the following antibodies: anti-CK5 (ab52635, Abcam, Cambridge, MA, USA), anti-CK14 (RB-9020, Thermo Scientific, Fremont, CA, USA), anti-CK8 (ab53280, Abcam), anti-estrogen receptor alpha (SC-542, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-PR (RM-9102, Thermo Scientific), anti-HER2/neu (MA5-15050, Thermo Scientific) and anti-vimentin (ab92547, Abcam). Images were obtained using an Olympus BX5.1 or BX41 microscope and Image Pro software. Hematoxylin and eosin-stained and immunostained slides were reviewed by a breast pathologist (JDM).
Protein preparation and western blot
Mammary tumors were lysed in ice-cold lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM NaVO4, protease inhibitor (11836170001, Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (P5726, Sigma-Aldrich, St Louis, MO, USA). An amount of 20 μg proteins were separated on 9% SDS-polyacrylamide gel electrophoresis gels and transferred to Immobilon-P membranes (IPVH00010, Millipore, Darmstadt, Germany). According to the standard protocols, the membranes were immunoblotted with following primary antibodies: anti-p110α (4249, Cell Signaling Technology, Beverley, CA, USA), anti-AKT1/2/3 (4691, Cell Signaling Technology), anti-p-AKT1/2/3 Ser473 (4060, Cell Signaling Technology), anti-p85α (sc-1637, Santa Cruz Biotechnology), anti-PTEN (9559, Cell Signaling Technology), anti-p-PTEN Ser380/Thr382/Thr383 (sc-101789, Santa Cruz Biotechnology), anti-GFP (sc-9996, Santa Cruz Biotechnology) and horseradish peroxidase-conjugated anti-β-Actin (ab49900, Abcam). This was followed by incubation with horseradish peroxidase-conjugated anti-rabbit (sc-2313, Santa Cruz Biotechnology) or anti-mouse (sc-2060, Santa Cruz Biotechnology) secondary antibody. Immunoreactive proteins were detected by chemiluminescence ECL (sc-2048, Santa Cruz Biotechnology), quantified using ImageLab software (Bio-Rad, Hercules, CA, USA) and normalized with respect to β-actin expression.
Preparation of breast tumor cells and assay of metastatic potential
The cells were derived from culturing large numbers of dissociated breast tumor cells in complete DMEM over several passages (less than five). The metastatic potential of breast tumor cells was tested by intravenous injection of 0.5 × 106 cells into the tail vein of syngeneic mice. The number of tumor lesions over 1.5 mm in diameter on the lungs was counted macroscopically after stained with 0.05% 1,9-dimethyl-methylene blue.
in vivo intratracheal delivery of Adeno-Cre virus into the lung
Six- to eight-week-old mice were anesthetized by intraperitoneal injection of avertin (0.3 mg/g body weight). The Exel Safelet IV catheter was inserted into the mouth and slid into the trachea, and the calcium phosphate precipitate containing 2.5 × 107 plaque-forming unit of Ad-Cre virus was injected through the opening of the catheter using a 0.5-ml syringe. Mice were monitored for clinical symptoms of poor health, such as lethargy, panting/respiratory distress and/or decreased mobility.
Mouse embryonic fibroblasts (MEFs) preparation
MEFs were derived and cultured as previously described.22
Ad-Cre virus infection of MEFs
60–70% confluent MEFs at passage number 2–3 were infected at multiplicity of infection 100:1 (virus:cell) with adenovirus-expressing Cre-recombinase (Ad5CMVCre, University of Iowa, Gene Transfer Vector Core) in DMEM supplemented with 2% fetal bovine serum for 6 h. The infection efficiency was confirmed by flow cytometry of eGFP-positive cells to ensure that >85% cells expressed the myr-p110α.
Anchorage-independent cell growth assay in vitro
A total of 2000 pooled transfected MEFs were resuspended in a top layer of 0.35% agar-containing DMEM and plated onto a bottom layer of 0.5% agar-containing DMEM in 12-well plates in triplicate. After 30 days, the colonies were stained with 0.005% crystal violet and counted macroscopically. Minimum diameter of the colonies was 50 μm.
In vivo tumor formation assay
Total 1 × 106 pooled transfected MEFs were injected intradermally into six- to eight-week-old immunocompromised NOD/scid IL2 gamma chain KO (NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice. Mice were monitored daily to detect a palpable/visible tumor.
Statistical analysis
Two-tailed unpaired Student’s t-test and the Mann–Whitney test were used for comparison between experimental groups analyzed for anchorage-independent cell growth assay, metastatic potential assay and western blots. For experiments involving a comparison between three or more distinct groups, one-way analysis of variance test was used. Statistics were calculated as non-parametric Log-rank (Mantel–Cox) analysis of Kaplan–Meier for censored survival and tumor-free data. Data analysis was performed using Prism 5.0 software (GraphPad software, La Jolla, CA, USA). Graphs in figures denoted statistical significance of *P<0.05, **P<0.01, and ***P<0.001. P>0.05 was considered non-significant (ns).
References
Manning BD, Cantley LC . AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274.
Yuan TL, Cantley LC . PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510.
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304: 554.
Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007; 448: 439–444.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA 2007; 104: 7564–7569.
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008; 68: 6084–6091.
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012; 486: 395–399.
Walerych D, Napoli M, Collavin L, Del Sal G . The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 2012; 33: 2007–2017.
Samuels Y, Waldman T . Oncogenic mutations of PIK3CA in human cancers. Curr Top Microbiol Immunol 2010; 347: 21–41.
Klarenbeek S, van Miltenburg MH, Jonkers J . Genetically engineered mouse models of PI3K signaling in breast cancer. Mol Oncol 2013; 7: 146–164.
Samuels Y, Waldman T . Oncogenic mutations of PIK3CA in human cancers. Curr Top Microbiol Immunol 2011; 347: 21–41.
Lai YL, Mau BL, Cheng WH, Chen HM, Chiu HH, Tzen CY . PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Ann Surg Oncol 2008; 15: 1064–1069.
Li SY, Rong M, Grieu F, Iacopetta B . PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006; 96: 91–95.
Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res 2009; 15: 5049–5059.
Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci USA 2010; 107: 10208–10213.
Miron A, Varadi M, Carrasco D, Li H, Luongo L, Kim HJ et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010; 70: 5674–5678.
Adams JR, Xu K, Liu JC, Agamez NM, Loch AJ, Wong RG et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res 2011; 71: 2706–2717.
Meyer DS, Brinkhaus H, Muller U, Muller M, Cardiff RD, Bentires-Alj M . Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res 2011; 71: 4344–4351.
Yuan W, Stawiski E, Janakiraman V, Chan E, Durinck S, Edgar KA et al. Conditional activation of Pik3ca(H1047R) in a knock-in mouse model promotes mammary tumorigenesis and emergence of mutations. Oncogene 2013; 32: 318–326.
Tikoo A, Roh V, Montgomery KG, Ivetac I, Waring P, Pelzer R et al. Physiological levels of Pik3ca(H1047R) mutation in the mouse mammary gland results in ductal hyperplasia and formation of ERalpha-positive tumors. PLoS ONE 2012; 7: e36924.
Renner O, Blanco-Aparicio C, Grassow M, Canamero M, Leal JF, Carnero A . Activation of phosphatidylinositol 3-kinase by membrane localization of p110alpha predisposes mammary glands to neoplastic transformation. Cancer Res 2008; 68: 9643–9653.
Sheen MR, Warner SL, Fields JL, Conejo-Garcia JR, Fiering S . Myristoylated p110α causes embryonic death due to developmental and vascular defects. Open Life Sci 2015; 10: 461–478.
Rutkowski MR, Allegrezza MJ, Svoronos N, Tesone AJ, Stephen TL, Perales-Puchalt A et al. Initiation of metastatic breast carcinoma by targeting of the ductal epithelium with adenovirus-cre: a novel transgenic mouse model of breast cancer. J Vis Exp 2014; 85: 51171.
Langerod A, Zhao H, Borgan O, Nesland JM, Bukholm IR, Ikdahl T et al. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res 2007; 9: R30.
Chalhoub N, Baker SJ . PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 2009; 4: 127–150.
Birle D, Bottini N, Williams S, Huynh H, deBelle I, Adamson E et al. Negative feedback regulation of the tumor suppressor PTEN by phosphoinositide-induced serine phosphorylation. J Immunol 2002; 169: 286–291.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1999; 96: 2110–2115.
Dunlap J, Le C, Shukla A, Patterson J, Presnell A, Heinrich MC et al. Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat 2010; 120: 409–418.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001; 15: 3243–3248.
Cespedes MV, Sancho FJ, Guerrero S, Parreno M, Casanova I, Pavon MA et al. K-ras Asp12 mutant neither interacts with Raf, nor signals through Erk and is less tumorigenic than K-ras Val12. Carcinogenesis 2006; 27: 2190–2200.
Rashid OM, Nagahashi M, Ramachandran S, Dumur CI, Schaum JC, Yamada A et al. Is tail vein injection a relevant breast cancer lung metastasis model? J Thorac Dis 2013; 5: 385–392.
Guille A, Chaffanet M, Birnbaum D . Signaling pathway switch in breast cancer. Cancer Cell Int 2013; 13: 66.
Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS . Breast cancer intra-tumor heterogeneity. Breast Cancer Res 2014; 16: 210.
Ng CK, Pemberton HN, Reis-Filho JS . Breast cancer intratumor genetic heterogeneity: causes and implications. Expert Rev Anticancer Ther 2012; 12: 1021–1032.
Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med 2011; 17: 1116–1120.
Sharma S, Kelly TK, Jones PA . Epigenetics in cancer. Carcinogenesis 2010; 31: 27–36.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A . Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 2001; 29: 418–425.
Acknowledgements
We acknowledge the assistance of the Dartmouth-Hitchcock Medical Center Laboratory for Clinical Genomics and Advanced Technology for immunohistochemistry services, the Dartmouth Mouse Modeling Shared Resource and DartMouse Speed Congenics Shared Resource for animal research support. Work was supported by NIH grants: Dartmouth Immunology COBRE 5P30GM103415-03 (S.F.), NIH U54 CA151662-01 (S.F.), Norris Cotton Cancer Center Support Grant P30 CA023108-27 (S.F.), R01CA124515 (J.R.C-G.), R01CA178687 (J.R.C-G.), R01CA157664 (J.R.C-G.), P30CA10815 (J.R.C-G.), and The Jayne Koskinas & Ted Giovanis Breast Cancer Research Consortium at Wistar.
Author contributions
MRS, JRC-G and SF conceived and designed the experiments. MRS performed the experiments, acquired the data, analyzed and interpreted the data, and wrote the manuscript. JDM reviewed hematoxylin and eosin-stained and immunostained slides for histopathological evaluation. MJA, MR and JRC-G provided the reagents and made intellectual contributions to the manuscript. All authors commented on the manuscript and gave final approval of the version to be submitted and any revised version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogenesis website .
Rights and permissions
Oncogenesis is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sheen, M., Marotti, J., Allegrezza, M. et al. Constitutively activated PI3K accelerates tumor initiation and modifies histopathology of breast cancer. Oncogenesis 5, e267 (2016). https://doi.org/10.1038/oncsis.2016.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2016.65
This article is cited by
-
Identification of PIK3CA multigene mutation patterns associated with superior prognosis in stomach cancer
BMC Cancer (2021)
-
Treatment response to idelalisib in a patient with immunodeficiency-associated Burkitt lymphoma harboring a PIK3CA H1047R mutation
Annals of Hematology (2021)
-
Excess active P13K rescues huntingtin-mediated neuronal cell death but has no effect on axonal transport defects
Apoptosis (2019)