Abstract
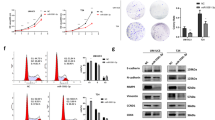
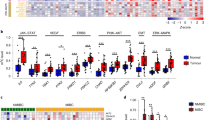
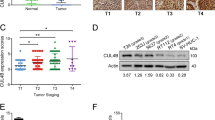
We have recently identified and characterized a novel oncogene, maelstrom (MAEL) from 1q24, in the pathogenesis of hepatocellular carcinoma. In this study, MAEL was investigated for its oncogenic role in urothelial carcinoma of the bladder (UCB) tumorigenesis/aggressiveness and underlying molecular mechanisms. Here, we report that overexpression of MAEL in UCB is important in the acquisition of an aggressive and/or poor prognostic phenotype. In UCB cell lines, knockdown of MAEL by short hairpin RNA is sufficient to inhibit cell growth, invasiveness/metastasis and suppressed epithelial–mesenchymal transition (EMT), whereas ectopic overexpression of MAEL promoted cell growth, invasive and/or metastatic capacity and enhanced EMT both in vitro and in vivo. We further demonstrate that MAEL could induce UCB cell EMT by downregulating a critical downstream target, the metastasis suppressor 1 (MTSS1) gene, ultimately leading to an increased invasiveness of cancer cells. Notably, overexpression of MAEL in UCB cells substantially enhanced the enrichment of DNA methyltrans-ferase (DNMT)3B and histone deacetylase (HDAC)1/2 on the promoter of the MTSS1, and thereby epigenetically suppressing the MTSS1 transcription. Downregulation of MTSS1 by MAEL in UCB cells is partially dependent on DNMT3B. Furthermore, we identify that beside the gene amplification of MAEL, miR-186 is a key negative regulator of MAEL and downregulation of miR-186 is another important mechanism for MAEL overexpression in UCBs. These data suggest that overexpression of MAEL, caused by gene amplification and/or decreased miR-186, has a critical oncogenic role in UCB pathogenesis by downregulation of MTSS1, and MAEL could be used as a novel prognostic marker and/or effective therapeutic target for human UCB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63: 234–241.
Malkowicz SB, van Poppel H, Mickisch G, Pansadoro V, Thuroff J, Soloway MS et al. Muscle-invasive urothelial carcinoma of the bladder. Urology 2007; 69: 3–16.
Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF et al. ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol 2013; 63: 58–66.
Hopman AH, Moesker O, Smeets AW, Pauwels RP, Vooijs GP, Ramaekers FC . Numerical chromosome 1, 7, 9, and 11 aberrations in bladder cancer detected by in situ hybridization. Cancer Res 1991; 51: 644–651.
Poddighe PJ, Ramaekers FC, Smeets AW, Vooijs GP, Hopman AH . Structural chromosome 1 aberrations in transitional cell carcinoma of the bladder: interphase cytogenetics combining a centromeric, telomeric, and library DNA probe. Cancer Res 1992; 52: 4929–4934.
Lopez V, Gonzalez-Peramato P, Suela J, Serrano A, Algaba F, Cigudosa JC et al. Identification of prefoldin amplification (1q23.3-q24.1) in bladder cancer using comparative genomic hybridization (CGH) arrays of urinary DNA. J Transl Med 2013; 11: 182.
Ma NF, Hu L, Fung JM, Xie D, Zheng BJ, Chen L et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology (Baltimore, MD) 2008; 47: 503–510.
Ma J, Gao M, Lu Y, Feng X, Zhang J, Lin D et al. Gain of 1q25-32, 12q23-24.3, and 17q12-22 facilitates tumorigenesis and progression of human squamous cell lung cancer. J Pathol 2006; 210: 205–213.
Zhou CZ, Qiu GQ, Fan JW, Wang XL, Tang HM, Huang L et al. Refined mapping of loss of heterozygosity on 1q31.1-32.1 in sporadic colorectal carcinoma. World J Gastroenterol 2008; 14: 1582–1587.
Mesquita B, Lopes P, Rodrigues A, Pereira D, Afonso M, Leal C et al. Frequent copy number gains at 1q21 and 1q32 are associated with overexpression of the ETS transcription factors ETV3 and ELF3 in breast cancer irrespective of molecular subtypes. Breast Cancer Res Treat 2013; 138: 37–45.
Maru DM, Luthra R, Correa AM, White-Cross J, Anandasabapathy S, Krishnan S et al. Frequent loss of heterozygosity of chromosome 1q in esophageal adenocarcinoma: loss of chromosome 1q21.3 is associated with shorter overall survival. Cancer 2009; 115: 1576–1585.
Liu L, Dai Y, Chen J, Zeng T, Li Y, Chen L et al. Maelstrom promotes hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition by way of Akt/GSK-3beta/Snail signaling. Hepatology 2014; 59: 531–543.
Clegg NJ, Frost DM, Larkin MK, Subrahmanyan L, Bryant Z, Ruohola-Baker H . Maelstrom is required for an early step in the establishment of Drosophila oocyte polarity: posterior localization of grk mRNA. Development (Cambridge, England) 1997; 124: 4661–4671.
Xiao L, Wang Y, Zhou Y, Sun Y, Sun W, Wang L et al. Identification of a novel human cancer/testis gene MAEL that is regulated by DNA methylation. Mol Biol Rep 2010; 37: 2355–2360.
Aravin AA, van der Heijden GW, Castaneda J, Vagin VV, Hannon GJ, Bortvin A . Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet 2009; 5: e1000764.
Matsumoto N, Sato K, Nishimasu H, Namba Y, Miyakubi K, Dohmae N et al. Crystal structure and activity of the endoribonuclease domain of the piRNA pathway factor maelstrom. Cell Rep 2015; 11: 366–375.
Cheng J, Deng H, Xiao B, Zhou H, Zhou F, Shen Z et al. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett 2012; 315: 12–17.
Lim SL, Ricciardelli C, Oehler MK, Tan IM, Russell D, Grutzner F . Overexpression of piRNA pathway genes in epithelial ovarian cancer. PloS One 2014; 9: e99687.
Fan H, Chen L, Zhang F, Quan Y, Su X, Qiu X et al. MTSS1, a novel target of DNA methyltransferase 3B, functions as a tumor suppressor in hepatocellular carcinoma. Oncogene 2012; 31: 2298–2308.
Cai Y, Geutjes EJ, de Lint K, Roepman P, Bruurs L, Yu LR et al. The NuRD complex cooperates with DNMTs to maintain silencing of key colorectal tumor suppressor genes. Oncogene 2014; 33: 2157–2168.
Stark GR, Debatisse M, Giulotto E, Wahl GM . Recent progress in understanding mechanisms of mammalian DNA amplification. Cell 1989; 57: 901–908.
He LR, Liu MZ, Li BK, Jia WH, Zhang Y, Liao YJ et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer 2010; 127: 138–147.
Yoshino H, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Nishiyama K et al. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer 2011; 104: 808–818.
Yao K, He L, Gan Y, Zeng Q, Dai Y, Tan J . MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn Pathol 2015; 10: 146.
Du P, Ye L, Ruge F, Yang Y, Jiang WG . Metastasis suppressor-1, MTSS1, acts as a putative tumour suppressor in human bladder cancer. Anticancer Res 2011; 31: 3205–3212.
Nixdorf S, Grimm MO, Loberg R, Marreiros A, Russell PJ, Pienta KJ et al. Expression and regulation of MIM (missing in metastasis), a novel putative metastasis suppressor gene, and MIM-B, in bladder cancer cell lines. Cancer Lett 2004; 215: 209–220.
Kayser G, Csanadi A, Kakanou S, Prasse A, Kassem A, Stickeler E et al. Downregulation of MTSS1 expression is an independent prognosticator in squamous cell carcinoma of the lung. Br J Cancer 2015; 112: 866–873.
Loberg RD, Neeley CK, Adam-Day LL, Fridman Y St, John LN, Nixdorf S et al. Differential expression analysis of MIM (MTSS1) splice variants and a functional role of MIM in prostate cancer cell biology. Int J Oncol 2005; 26: 1699–1705.
Liu K, Wang G, Ding H, Chen Y, Yu G, Wang J . Downregulation of metastasis suppressor 1(MTSS1) is associated with nodal metastasis and poor outcome in Chinese patients with gastric cancer. BMC Cancer 2010; 10: 428.
Mertz KD, Pathria G, Wagner C, Saarikangas J, Sboner A, Romanov J et al. MTSS1 is a metastasis driver in a subset of human melanomas. Nat Commun 2014; 5: 3465.
Giacobbe A, Compagnone M, Bongiorno-Borbone L, Antonov A, Markert EK, Zhou JH et al. p63 controls cell migration and invasion by transcriptional regulation of MTSS1. Oncogene 2015; 35: 1602–1608.
Bachman KE, Rountree MR, Baylin SB . Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J Biol Chem 2001; 276: 32282–32287.
Rountree MR, Bachman KE, Baylin SB . DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet 2000; 25: 269–277.
Fuks F, Burgers WA, Godin N, Kasai M, Kouzarides T . Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J 2001; 20: 2536–2544.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297.
Kottakis F, Polytarchou C, Foltopoulou P, Sanidas I, Kampranis SC, Tsichlis PN . FGF-2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B-miR-101-EZH2 pathway. Mol Cell 2011; 43: 285–298.
Zheng F, Liao YJ, Cai MY, Liu TH, Chen SP, Wu PH et al. Systemic delivery of microRNA-101 potently inhibits hepatocellular carcinoma in vivo by repressing multiple targets. PLoS Genet 2015; 11: e1004873.
Xie D, Sham JS, Zeng WF, Lin HL, Bi J, Che LH et al. Correlation of AIB1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum Pathol 2005; 36: 777–783.
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 2012; 31: 583–594.
Acknowledgements
This work was supported by grants from the Nature Science Foundation of China (no. 81225018 and no.81472385), the Fundamental Research Funds for the Central Universities (Sun Yat-Sen University Young Teachers Plan) (no. 14ykpy39), Natural Science Foundation of Guangdong Province (no. 2015A030313009), China and Science and Technology Foundation of the Guangdong Province, China (grant no. 2013B021800173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, XD., Zhang, JX., Jiang, LJ. et al. Overexpression of maelstrom promotes bladder urothelial carcinoma cell aggressiveness by epigenetically downregulating MTSS1 through DNMT3B. Oncogene 35, 6281–6292 (2016). https://doi.org/10.1038/onc.2016.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.165
This article is cited by
-
Maelstrom promotes tumor metastasis through regulation of FGFR4 and epithelial-mesenchymal transition in epithelial ovarian cancer
Journal of Ovarian Research (2022)
-
MAEL as a diagnostic marker for the early detection of esophageal squamous cell carcinoma
Diagnostic Pathology (2021)
-
MAEL Cancer-Testis Antigen as a Diagnostic Marker in Primary Stages of Gastric Cancer with Helicobacter pylori Infection
Journal of Gastrointestinal Cancer (2020)
-
Computational analysis of the evolutionarily conserved Missing In Metastasis/Metastasis Suppressor 1 gene predicts novel interactions, regulatory regions and transcriptional control
Scientific Reports (2019)
-
N6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis
Nature Communications (2019)