Abstract
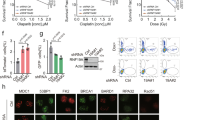
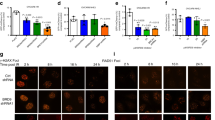
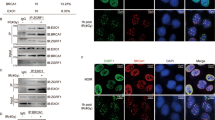
RNF126 is an E3 ubiquitin ligase. The deletion of RNF126 gene was observed in a wide range of human cancers and is correlated with improved disease-free and overall survival. These data highlight the clinical relevance of RNF126 in tumorigenesis and cancer therapy. However, the specific functions of RNF126 remain largely unknown. Homologous recombination (HR)-mediated DNA double-strand break repair is important for tumor suppression and cancer therapy resistance. Here, we demonstrate that RNF126 facilitates HR by promoting the expression of BRCA1, in a manner independent of its E3 ligase activity but depending on E2F1, a well-known transcription factor of BRCA1 promoter. In support of this result, RNF126 promotes transactivation of BRCA1 promoter by directly binding to E2F1. Most importantly, an RNF126 mutant lacking 11 amino acids that is responsible for the interaction with E2F1 has a dominant-negative effect on BRCA1 expression and HR by suppressing E2F1-mediated transactivation of BRCA1 promoter and blocking the enrichment of E2F1 on BRCA1 promoter. Lastly, RNF126 depletion leads to the increased sensitivity to ionizing radiation and poly (ADP-ribose) polymerase inhibition. Collectively, our results suggest a novel role of RNF126 in promoting HR-mediated repair through positive regulation on BRCA1 expression by direct interaction with E2F1. This study not only offers novel insights into our current understanding of the biological functions of RNF126 but also provides a potential therapeutic target for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, Ogawa H et al. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J 1998; 17: 598–608.
Andreassen PR, Ho GP, D'Andrea AD . DNA damage responses and their many interactions with the replication fork. Carcinogenesis 2006; 27: 883–892.
Evers B, Jonkers J . Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene 2006; 25: 5885–5897.
Zhang J . The role of BRCA1 in homologous recombination repair in response to replication stress: significance in tumorigenesis and cancer therapy. Cell Biosci 2013; 3: 11.
Moynahan ME, Jasin M . Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol 2010; 11: 196–207.
Helleday T . Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis 2010; 31: 955–960.
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921.
Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT . Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet 1995; 9: 444–450.
Russell PA, Pharoah PD, De Foy K, Ramus SJ, Symmonds I, Wilson A et al. Frequent loss of BRCA1 mRNA and protein expression in sporadic ovarian cancers. Int J Cancer 2000; 87: 317–321.
Zheng W, Luo F, Lu JJ, Baltayan A, Press MF, Zhang ZF et al. Reduction of BRCA1 expression in sporadic ovarian cancer. Gynecol Oncol 2000; 76: 294–300.
Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2007; 26: 2126–2132.
Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet 1999; 21: 236–240.
Mueller CR, Roskelley CD . Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Res 2003; 5: 45–52.
Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res 2005; 65: 11597–11604.
De Siervi A, De Luca P, Byun JS, Di LJ, Fufa T, Haggerty CM et al. Transcriptional autoregulation by BRCA1. Cancer Res 2010; 70: 532–542.
Rice JC, Massey-Brown KS, Futscher BW . Aberrant methylation of the BRCA1 CpG island promoter is associated with decreased BRCA1 mRNA in sporadic breast cancer cells. Oncogene 1998; 17: 1807–1812.
Johnson DG, Degregori J . Putting the oncogenic and tumor suppressive activities of E2F into context. Curr Mol Med 2006; 6: 731–738.
Biswas AK, Johnson DG . Transcriptional and nontranscriptional functions of E2F1 in response to DNA damage. Cancer Res 2012; 72: 13–17.
Chen J, Zhu F, Weaks RL, Biswas AK, Guo R, Li Y et al. E2F1 promotes the recruitment of DNA repair factors to sites of DNA double-strand breaks. Cell Cycle 2011; 10: 1287–1294.
Biswas AK, Mitchell DL, Johnson DG . E2F1 responds to ultraviolet radiation by directly stimulating DNA repair and suppressing carcinogenesis. Cancer Res 2014; 74: 3369–3377.
Putzer BM, Engelmann D . E2F1 apoptosis counterattacked: evil strikes back. Trends Mol Med 2013; 19: 89–98.
Wang A, Schneider-Broussard R, Kumar AP, MacLeod MC, Johnson DG . Regulation of BRCA1 expression by the Rb-E2F pathway. J Biol Chem 2000; 275: 4532–4536.
Iwanaga R, Komori H, Ohtani K . Differential regulation of expression of the mammalian DNA repair genes by growth stimulation. Oncogene 2004; 23: 8581–8590.
Bindra RS, Glazer PM . Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene 2007; 26: 2048–2057.
Stanelle J, Stiewe T, Theseling CC, Peter M, Putzer BM . Gene expression changes in response to E2F1 activation. Nucleic Acids Res 2002; 30: 1859–1867.
Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 2002; 16: 245–256.
Guo R, Chen J, Zhu F, Biswas AK, Berton TR, Mitchell DL et al. E2F1 localizes to sites of UV-induced DNA damage to enhance nucleotide excision repair. J Biol Chem 2010; 285: 19308–19315.
Liu K, Lin FT, Ruppert JM, Lin WC . Regulation of E2F1 by BRCT domain-containing protein TopBP1. Mol Cell Biol 2003; 23: 3287–3304.
Zhi X, Zhao D, Wang Z, Zhou Z, Wang C, Chen W et al. E3 ubiquitin ligase RNF126 promotes cancer cell proliferation by targeting the tumor suppressor p21 for ubiquitin-mediated degradation. Cancer Res 2012; 73: 385–394.
Smith CJ, Berry DM, McGlade CJ . The E3 ubiquitin ligases RNF126 and Rabring7 regulate endosomal sorting of the epidermal growth factor receptor. J Cell Sci 2013; 126 (Pt 6): 1366–1380.
Delker RK, Zhou Y, Strikoudis A, Stebbins CE, Papavasiliou FN . Solubility-based genetic screen identifies RING finger protein 126 as an E3 ligase for activation-induced cytidine deaminase. Proc Natl Acad Sci USA 2013; 110: 1029–1034.
Wang ZJ, Churchman M, Campbell IG, Xu WH, Yan ZY, McCluggage WG et al. Allele loss and mutation screen at the Peutz-Jeghers (LKB1) locus (19p13.3) in sporadic ovarian tumors. Br J Cancer 1999; 80: 70–72.
Yanaihara N, Okamoto A, Matsufuji S . A commonly deleted region in ovarian cancer on chromosome 19p13.3, not including the OAZ1 gene. Int J Oncol 2003; 23: 567–575.
Amfo K, Neyns B, Teugels E, Lissens W, Bourgain C, De Sutter P et al. Frequent deletion of chromosome 19 and a rare rearrangement of 19p13.3 involving the insulin receptor gene in human ovarian cancer. Oncogene 1995; 11: 351–358.
Yang TL, Su YR, Huang CS, Yu JC, Lo YL, Wu PE et al. High-resolution 19p13.2-13.3 allelotyping of breast carcinomas demonstrates frequent loss of heterozygosity. Genes Chromosomes Cancer 2004; 41: 250–256.
Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S et al. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell 2005; 8: 255–265.
Shi W, Feng Z, Zhang J, Gonzalez-Suarez I, Vanderwaal RP, Wu X et al. The role of RPA2 phosphorylation in homologous recombination in response to replication arrest. Carcinogenesis 2010; 31: 994–1002.
Chen H, Ma Z, Vanderwaal RP, Feng Z, Gonzalez-Suarez I, Wang S et al. The mTOR inhibitor rapamycin suppresses DNA double-strand break repair. Radiation Res 2011; 175: 214–224.
Zhang J, Ma Z, Treszezamsky A, Powell SN . MDC1 interacts with Rad51 and facilitates homologous recombination. Nat Struct Mol Biol 2005; 12: 902–909.
Pierce AJ, Johnson RD, Thompson LH, Jasin M . XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev 1999; 13: 2633–2638.
Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 1997; 88: 265–275.
Yang SZ, Lin FT, Lin WC . MCPH1/BRIT1 cooperates with E2F1 in the activation of checkpoint, DNA repair and apoptosis. EMBO Rep 2008; 9: 907–915.
Wang B, Liu K, Lin FT, Lin WC . A role for 14-3-3 tau in E2F1 stabilization and DNA damage-induced apoptosis. J Biol Chem 2004; 279: 54140–54152.
Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol 2003; 5: 552–558.
Guo R, Chen J, Mitchell DL, Johnson DG . GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res 2011; 39: 1390–1397.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Feng Z, Zhang J . A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest. Nucleic Acids Res 2012; 40: 726–738.
Zhuang J, Zhang J, Willers H, Wang H, Chung JH, van Gent DC et al. Checkpoint kinase 2-mediated phosphorylation of BRCA1 regulates the fidelity of nonhomologous end-joining. Cancer Res 2006; 66: 1401–1408.
Acknowledgements
This study was supported by a grant (R01C A154625) from the National Cancer Institute and a startup fund from the Department of Radiation Oncology, Case Western Reserve University School of Medicine to JZ; National Natural Science Foundation of China grant (31271503), Guangdong Provincial Natural Science Foundation of China grant (S2012010008368) and a startup fund from Sun Yat-sen University to ZM. This research was also supported by Radiation Resources Core Facility and Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center (P30 CA43703). We thank Daniel Li and Mehdi Haider for the technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Y., Deng, O., Feng, Z. et al. RNF126 promotes homologous recombination via regulation of E2F1-mediated BRCA1 expression. Oncogene 35, 1363–1372 (2016). https://doi.org/10.1038/onc.2015.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.198
This article is cited by
-
NCAPD3 enhances Warburg effect through c-myc and E2F1 and promotes the occurrence and progression of colorectal cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
Ring finger protein 126 promotes breast cancer metastasis and serves as a potential target to improve the therapeutic sensitivity of ATR inhibitors
Breast Cancer Research (2022)
-
E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability
Cell Death & Disease (2021)
-
Rewiring E2F1 with classical NHEJ via APLF suppression promotes bladder cancer invasiveness
Journal of Experimental & Clinical Cancer Research (2019)
-
Upregulation of DARS2 by HBV promotes hepatocarcinogenesis through the miR-30e-5p/MAPK/NFAT5 pathway
Journal of Experimental & Clinical Cancer Research (2017)