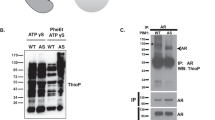
Abstract
Phosphatidylinositol 3-kinase (PI3K) promotes cancer cell survival, migration, growth and proliferation by generating phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the inner leaflet of the plasma membrane. PIP3 recruits pleckstrin homology domain-containing proteins to the membrane to activate oncogenic signaling cascades. Anticancer therapeutics targeting the PI3K/AKT/mTOR (mammalian target of rapamycin) pathway are in clinical development. In a mass spectrometric screen to identify PIP3-regulated proteins in breast cancer cells, levels of the Rac activator PIP3-dependent Rac exchange factor-1 (P-REX1) increased in response to PI3K inhibition, and decreased upon loss of the PI3K antagonist phosphatase and tensin homolog (PTEN). P-REX1 mRNA and protein levels were positively correlated with ER expression, and inversely correlated with PI3K pathway activation in breast tumors as assessed by gene expression and phosphoproteomic analyses. P-REX1 increased activation of Rac1, PI3K/AKT and MEK/ERK signaling in a PTEN-independent manner, and promoted cell and tumor viability. Loss of P-REX1 or inhibition of Rac suppressed PI3K/AKT and MEK/ERK, and decreased viability. P-REX1 also promoted insulin-like growth factor-1 receptor activation, suggesting that P-REX1 provides positive feedback to activators upstream of PI3K. In support of a model where PIP3-driven P-REX1 promotes both PI3K/AKT and MEK/ERK signaling, high levels of P-REX1 mRNA (but not phospho-AKT or a transcriptomic signature of PI3K activation) were predictive of sensitivity to PI3K inhibitors among breast cancer cell lines. P-REX1 expression was highest in estrogen receptor-positive breast tumors compared with many other cancer subtypes, suggesting that neutralizing the P-REX1/Rac axis may provide a novel therapeutic approach to selectively abrogate oncogenic signaling in breast cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others

References
Miller TW, Rexer BN, Garrett JT, Arteaga CL . Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011; 13: 224.
Engelman JA . Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009; 9: 550–562.
Whitman M, Downes CP, Keeler M, Keller T, Cantley L . Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 1988; 332: 644–646.
Maehama T, Dixon JE . The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–13378.
Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 2012; 11: 317–328.
Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell 2002; 108: 809–821.
Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J et al. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene 2009; 28: 1853–1863.
Montero JC, Seoane S, Ocana A, Pandiella A . P-Rex1 participates in neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer. Oncogene 2011; 30: 1059–1071.
Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell 2010; 40: 877–892.
Fine B, Hodakoski C, Koujak S, Su T, Saal LH, Maurer M et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science 2009; 325: 1261–1265.
Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 2008; 51: 5522–5532.
Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G et al. Loss of phosphatase and tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res 2009; 69: 4192–4201.
Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL . Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA 2011; 109: 2718–2723.
Khatri S, Yepiskoposyan H, Gallo CA, Tandon P, Plas DR . FOXO3a regulates glycolysis via transcriptional control of tumor suppressor TSC1. J Biol Chem 2010; 285: 15960–15965.
Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res 2010; 12: R40.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486: 346–352.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA 2007; 104: 7564–7569.
Hanker AB, Pfefferle AD, Balko JM, Kuba MG, Young CD, Sanchez V et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci USA 2013; 110: 14372–14377.
Yuan TL, Cantley LC . PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510.
Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 2013; 39: 935–946.
Wang ZP, Fu M, Wang LF, Liu JJ, Li YH, Brakebusch C et al. P21-activated kinase 1 (PAK1) can promote ERK. J Biol Chem 2013; 288: 20093–20099.
King AJ, Sun HY, Diaz B, Barnard D, Miao WY, Bagrodia S et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 1998; 396: 180–183.
Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol 2003; 162: 281–291.
Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L . A brain serine threonine protein-kinase activated by Cdc42 and Rac1. Nature 1994; 367: 40–46.
Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013; 153: 1050–1063.
Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH et al. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol Cell 2012; 47: 281–290.
Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ . Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Small Gtpases Dis Part B 2008; 439: 111–129.
Hill K, Krugmann S, Andrews SR, Coadwell WJ, Finan P, Welch HC et al. Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gbetagamma subunits. J Biol Chem 2005; 280: 4166–4173.
Hoffman GR, Cerione RA . Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett 2002; 513: 85–91.
Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Reyes-Cruz G et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem 2007; 282: 23708–23715.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012; 483: 603–607.
O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3' kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res 2010; 16: 3670–3683.
Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci USA 2013; 110: 21124–21129.
Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A et al. The p110 beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110 gamma. Proc Natl Acad Sci USA 2008; 105: 8292–8297.
Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 2010; 29: 5839–5849.
Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A . The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal 2004; 16: 1001–1011.
Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA 2011; 108: 7177–7182.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM et al. P-Rex1 regulates neutrophil function. Curr Biol 2005; 15: 1867–1873.
Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G et al. Loss of phosphatase and tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res 2009; 69: 4192–4201.
MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci USA 2002; 99: 7900–7905.
Martinez MN, Emfinger CH, Overton M, Hill S, Ramaswamy TS, Cappel DA et al. Obesity and altered glucose metabolism impact HDL composition in CETP transgenic mice: a role for ovarian hormones. J Lipid Res 2012; 53: 379–389.
Eng JK, McCormack AL, Yates JR III . An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 1994; 5: 976–989.
Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ et al. IDPicker 2.0: improved protein assembly with high discrimination peptide identification filtering. J Proteome Res 2009; 8: 3872–3881.
Li J, Lu Y, Akbani R, Ju Z, Roebuck PL, Liu W et al. TCPA: a resource for cancer functional proteomics data. Nat Methods 2013; 10: 1046–1047.
Hu ZY, Fan C, Oh DS, Marron JS, He XP, Qaqish BF et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genom 2006; 7: 96.
Acknowledgements
We thank Chad Creighton for advice on gene expression analysis, and the Norris Cotton Cancer Center Transgenic and Genetic Construct Shared Resource and Genomics and Molecular Biology Shared Resource for assistance. This work was financially supported by NIH: K99/R00CA142899 (TWM), Dartmouth College Norris Cotton Cancer Center Support Grant P30CA023108, Vanderbilt-Ingram Cancer Center Breast Cancer Specialized Program of Research Excellence Grant P50CA98131 and Vanderbilt-Ingram Cancer Center Support Grant P30CA68485; the American Cancer Society IRG-58-009-50 (TWM) and 121329-RSG-11-187-01-TBG (to AMG); and The Commonwealth Foundation for Cancer Research (to AMG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Dillon, L., Bean, J., Yang, W. et al. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene 34, 3968–3976 (2015). https://doi.org/10.1038/onc.2014.328
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.328
This article is cited by
-
Data mining-based identification of epigenetic signatures with discrimination potential of lung adenocarcinoma and squamous cell carcinoma
Molecular Biology Reports (2024)
-
HER2 low expression breast cancer subtyping and their correlation with prognosis and immune landscape based on the histone modification related genes
Scientific Reports (2023)
-
The pseudokinase NRBP1 activates Rac1/Cdc42 via P-Rex1 to drive oncogenic signalling in triple-negative breast cancer
Oncogene (2023)
-
MUC1 is a potential target to overcome trastuzumab resistance in breast cancer therapy
Cancer Cell International (2022)
-
Coffee decoction enhances tamoxifen proapoptotic activity on MCF-7 cells
Scientific Reports (2020)