Abstract
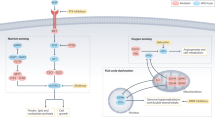
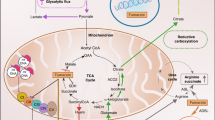
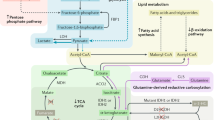
Cancer-associated mutations have been identified in the metabolic genes succinate dehydrogenase (SDH), fumarate hydratase (FH) and isocitrate dehydrogenase (IDH), advancing and challenging our understanding of cellular function and disease mechanisms and providing direct links between dysregulated metabolism and cancer. Some striking parallels exist in the cellular consequences of the genetic mutations within this triad of cancer syndromes, including accumulation of oncometabolites and competitive inhibition of 2-oxoglutarate-dependent dioxygenases, particularly, hypoxia-inducible factor (HIF) prolyl hydroxylases, JmjC domain-containing histone demethylases (part of the JMJD family) and the ten-eleven translocation (TET) family of 5methyl cytosine (5mC) DNA hydroxylases. These lead to activation of HIF-dependent oncogenic pathways and inhibition of histone and DNA demethylation. Mutations in FH, resulting in loss of enzyme activity, predispose affected individuals to a rare cancer, hereditary leiomyomatosis and renal cell cancer (HLRCC), characterised by benign smooth muscle cutaneous and uterine tumours (leiomyomata) and an aggressive form of collecting duct and type 2 papillary renal cancer. Interestingly, loss of FH activity results in the accumulation of high levels of fumarate that can lead to the non-enzymatic modification of cysteine residues in multiple proteins (succination) and in some cases to their disrupted function. Here we consider that the study of rare diseases such as HLRCC, combining analyses of human tumours and cell lines with in vitro and in vivo murine models has provided novel insights into cancer biology associated with dysregulated metabolism and represents a useful paradigm for cancer research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Warburg O . On respiratory impairment in cancer cells. Science 1956; 124: 269–270.
Warburg O . On the origin of cancer cells. Science 1956; 123: 309–314.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed 2012; 25: 1234–1244.
Ashcroft FM, Rorsman P . Diabetes mellitus and the beta cell: the last ten years. Cell 2012; 148: 1160–1171.
Semenza GL . Oxygen sensing, homeostasis, and disease. N Engl J Med 2011; 365: 537–547.
Kaelin WG Jr, Ratcliffe PJ . Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402.
DeBerardinis RJ, Thompson CB . Cellular metabolism and disease: what do metabolic outliers teach us? Cell 2012; 148: 1132–1144.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Schulze A, Harris AL . How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012; 491: 364–373.
Thompson CB . Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med 2009; 360: 813–815.
Frezza C, Pollard PJ, Gottlieb E . Inborn and acquired metabolic defects in cancer. J Mol Med (Berl) 2011; 89: 213–220.
Tomita M, Kami K . Cancer. Systems biology, metabolomics, and cancer metabolism. Science 2012; 336: 990–991.
Glunde K, Bhujwalla ZM, Ronen SM . Choline metabolism in malignant transformation. Nat Rev Cancer 2011; 11: 835–848.
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012; 18: 624–629.
Choi C, Ganji SK, DeBerardinis RJ, Dimitrov IE, Pascual JM, Bachoo R et al. Measurement of glycine in the human brain in vivo by 1H-MRS at 3 T: application in brain tumors. Magn Reson Med 2011; 66: 609–618.
Pollard PJ, Spencer-Dene B, Shukla D, Howarth K, Nye E, El-Bahrawy M et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell 2007; 11: 311–319.
Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA et al. UOK 262 cell line, fumarate hydratase deficient (FH-/FH-) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet 2010; 196: 45–55.
Bayley JP, van Minderhout I, Hogendoorn PC, Cornelisse CJ, van der Wal A, Prins FA et al. Sdhd and SDHD/H19 knockout mice do not develop paraganglioma or pheochromocytoma. PLoS One 2009; 4: e7987.
Piruat JI, Pintado CO, Ortega-Saenz P, Roche M, Lopez-Barneo J . The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol Cell Biol 2004; 24: 10933–10940.
Yang Y, Valera V, Sourbier C, Vocke CD, Wei M, Pike L et al. A novel fumarate hydratase-deficient HLRCC kidney cancer cell line, UOK268: a model of the Warburg effect in cancer. Cancer Genet 2012; 205: 377–390.
MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol 2007; 27: 3282–3289.
Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 2005; 8: 143–153.
Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 2012; 488: 656–659.
Sasaki M, Knobbe CB, Itsumi M, Elia AJ, Harris IS, Chio II et al. D-2-Hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev 2012; 26: 2038–2049.
Ternette N, Yang M, Laroyia M, Kitagawa M, O’Flaherty L, Wolhulter K et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep 2013; 3: 689–700.
Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 2011; 20: 524–537.
Soga T, Igarashi K, Ito C, Mizobuchi K, Zimmermann HP, Tomita M . Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem 2009; 81: 6165–6174.
Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem 2006; 281: 16768–16776.
Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T . Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res 2003; 2: 488–494.
Stratton MR . Journeys into the genome of cancer cells. EMBO Mol Med 2013; 5: 169–172.
Bayley JP, Devilee P . Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes barking up the right tree? Curr Opin Genet Dev 2010; 20: 324–329.
Knudson AG Jr . Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971; 68: 820–823.
Gottlieb E, Tomlinson IP . Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer 2005; 5: 857–866.
Ward PS, Thompson CB . Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21: 297–308.
Salway JG . Metabolism at a Glance (At a Glance (Blackwell)). Blackwell Publishers, Oxford, UK, 1999.
Bardella C, Pollard PJ, Tomlinson I . SDH mutations in cancer. Biochim Biophys Acta 2011; 1807: 1432–1443.
Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000; 287: 848–851.
Lenders JW, Eisenhofer G, Mannelli M, Pacak K . Phaeochromocytoma. Lancet 2005; 366: 665–675.
Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 2004; 74: 153–159.
Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 2010; 19: 3011–3020.
Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009; 325: 1139–1142.
Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001; 69: 49–54.
Niemann S, Muller U . Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 2000; 26: 268–270.
Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 2008; 100: 1260–1262.
Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA 2011; 108: 314–318.
Pasini B, Stratakis CA . SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med 2009; 266: 19–42.
Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res 2003; 63: 5615–5621.
Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 2007; 92: 3822–3828.
Young RM, Simon MC . Untuning the tumor metabolic machine: HIF-alpha: pro- and antitumorigenic? Nat Med 2012; 18: 1024–1025.
Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 2005; 14: 2231–2239.
Briere JJ, Favier J, Benit P, El Ghouzzi V, Lorenzato A, Rabier D et al. Mitochondrial succinate is instrumental for HIF1alpha nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Hum Mol Genet 2005; 14: 3263–3269.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005; 7: 77–85.
Dahia PL . Transcription association of VHL and SDH mutations link hypoxia and oxidoreductase signals in pheochromocytomas. Ann N Y Acad Sci 2006; 1073: 208–220.
Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet 2005; 1: 72–80.
Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet 2001; 69: 1186–1197.
Kaelin WG Jr . SDH5 mutations and familial paraganglioma: somewhere Warburg is smiling. Cancer Cell 2009; 16: 180–182.
Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT . Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 2008; 28: 718–731.
Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B . The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol 2007; 293: C12–C21.
Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N . A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res 2005; 65: 203–209.
Shi Y . Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet 2007; 8: 829–833.
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324: 930–935.
Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333: 1300–1303.
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y . Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466: 1129–1133.
Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev 2012; 26: 1326–1338.
Tan AY, Manley JL . The TET family of proteins: functions and roles in disease. J Mol Cell Biol 2009; 1: 82–92.
Varier RA, Timmers HT . Histone lysine methylation and demethylation pathways in cancer. Biochim Biophys Acta 2011; 1815: 75–89.
Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010; 463: 360–363.
van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet 2009; 41: 521–523.
Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 2010; 468: 839–843.
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP . CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999; 96: 8681–8686.
Hanan EJ, van Abbema A, Barrett K, Blair WS, Blaney J, Chang C et al. Discovery of potent and selective pyrazolopyrimidine janus kinase 2 inhibitors. J Med Chem 2012; 55: 10090–10107.
Rawson JB, Sun Z, Dicks E, Daftary D, Parfrey PS, Green RC et al. Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients. Nutr Cancer 2012; 64: 919–928.
Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 2011; 96: E1472–E1476.
van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol 2009; 10: 764–771.
Leonardi R, Subramanian C, Jackowski S, Rock CO . Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem 2012; 287: 14615–14620.
Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 2010; 28: 2348–2355.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360: 765–773.
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009; 361: 1058–1066.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–1812.
Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood 2012; 119: 1901–1903.
Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012; 17: 72–79.
Shibata T, Kokubu A, Miyamoto M, Sasajima Y, Yamazaki N . Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am J Pathol 2011; 178: 1395–1402.
Pansuriya TC, van Eijk R, d’Adamo P, van Ruler MA, Kuijjer ML, Oosting J et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet 2011; 43: 1256–1261.
Amary MF, Damato S, Halai D, Eskandarpour M, Berisha F, Bonar F et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet 2011; 43: 1262–1265.
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 2011; 224: 334–343.
Murugan AK, Bojdani E, Xing M . Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun 2010; 393: 555–559.
Hemerly JP, Bastos AU, Cerutti JM . Identification of several novel non-p.R132 IDH1 variants in thyroid carcinomas. Eur J Endocrinol 2010; 163: 747–755.
Gaal J, Burnichon N, Korpershoek E, Roncelin I, Bertherat J, Plouin PF et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 2010; 95: 1274–1278.
Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Seo SI et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer 2009; 125: 353–355.
Ward PS, Cross JR, Lu C, Weigert O, Abel-Wahab O, Levine RL et al. Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene 2012; 31: 2491–2498.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010; 465: 966.
Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012; 483: 479–483.
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012; 483: 474–478.
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011; 19: 17–30.
Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 2011; 12: 463–469.
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010; 17: 510–522.
Lorsbach RB, Moore J, Mathew S, Raimondi SC, Mukatira ST, Downing JR . TET1, a member of a novel protein family, is fused to MLL in acute myeloid leukemia containing the t(10;11)(q22;q23). Leukemia 2003; 17: 637–641.
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010; 18: 553–567.
Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 2012; 483: 484–488.
Losman JA, Looper R, Koivunen P, Lee S, Schneider RK, McMahon C et al. (R)-2-Hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013; 339: 1621–1625.
Stepinski J, Bizon D, Piec G, Angielski S . The purine nucleotide cycle activity in renal cortex and medulla. Am J Kidney Dis 1989; 14: 307–309.
Brosnan ME, Brosnan JT . Renal arginine metabolism. J Nutr 2004; 134 (10 Suppl): 2791S–2795SS discussion 6S-7S.
Yogev O, Naamati A, Pines O . Fumarase: a paradigm of dual targeting and dual localized functions. FEBS J 2011; 278: 4230–4242.
Yogev O, Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD et al. Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol 2010; 8: e1000328.
Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 2003; 73: 95–106.
Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002; 30: 406–410.
Kiuru M, Launonen V, Hietala M, Aittomaki K, Vierimaa O, Salovaara R et al. Familial cutaneous leiomyomatosis is a two-hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol 2001; 159: 825–829.
Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA 2001; 98: 3387–3392.
Linehan WM, Pinto PA, Srinivasan R, Merino M, Choyke P, Choyke L et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res 2007; 13 (2 Pt 2): 671s–679ss.
Ashrafian H, O’Flaherty L, Adam J, Steeples V, Chung YL, East P et al. Expression profiling in progressive stages of fumarate-hydratase deficiency: the contribution of metabolic changes to tumorigenesis. Cancer Res 2010; 70: 9153–9165.
Ratcliffe PJ . Fumarate hydratase deficiency and cancer: activation of hypoxia signaling? Cancer Cell 2007; 11: 303–305.
Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S et al. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol 2005; 205: 41–49.
Sudarshan S, Sourbier C, Kong HS, Block K, Valera Romero VA, Yang Y et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol 2009; 29: 4080–4090.
Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J . Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 2007; 282: 4524–4532.
Hewitson KS, Lienard BM, McDonough MA, Clifton IJ, Butler D, Soares AS et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem 2007; 282: 3293–3301.
Pollard PJ, Ratcliffe PJ . Cancer. Puzzling patterns of predisposition. Science 2009; 324: 192–194.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–275.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54.
Bruick RK, McKnight SL . A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001; 294: 1337–1340.
O’Flaherty L, Adam J, Heather LC, Zhdanov AV, Chung YL, Miranda MX et al. Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum Mol Genet 2010; 19: 3844–3851.
Yang M, Soga T, Pollard PJ, Adam J . The emerging role of fumarate as an oncometabolite. Front Oncol 2012; 2: 85.
Kaelin WG Jr . The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8: 865–873.
Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet 2006; 43: 523–526.
Adam J, Ratcliffe PJ, Pollard PJ . Novel insights into FH-associated disease are KEAPing the lid on oncogenic HIF signalling. Oncotarget 2011; 2: 820–821.
Frizzell N, Lima M, Baynes JW . Succination of proteins in diabetes. Free Radic Res 2011; 45: 101–109.
Nagai R, Brock JW, Blatnik M, Baatz JE, Bethard J, Walla MD et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem 2007; 282: 34219–34228.
Alderson NL, Wang Y, Blatnik M, Frizzell N, Walla MD, Lyons TJ et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys 2006; 450: 1–8.
Frizzell N, Rajesh M, Jepson MJ, Nagai R, Carson JA, Thorpe SR et al. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J Biol Chem 2009; 284: 25772–25781.
Blatnik M, Thorpe SR, Baynes JW . Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann N Y Acad Sci 2008; 1126: 272–275.
Blatnik M, Frizzell N, Thorpe SR, Baynes JW . Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes 2008; 57: 41–49.
Thomas SA, Storey KB, Baynes JW, Frizzell N . Tissue distribution of S-(2-succino)cysteine (2SC), a biomarker of mitochondrial stress in obesity and diabetes. Obesity (Silver Spring) 2012; 20: 263–269.
Maxwell PH . Seeing the smoking gun: a sensitive and specific method to visualize loss of the tumour suppressor, fumarate hydratase, in human tissues. J Pathol 2011; 225: 1–3.
Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol 2011; 225: 4–11.
Zhang DD . Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 2006; 38: 769–789.
Taguchi K, Motohashi H, Yamamoto M . Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011; 16: 123–140.
Hayes JD, McMahon M . NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 2009; 34: 176–188.
Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 2011; 20: 511–523.
Ooi A, Dykema K, Ansari A, Petillo D, Snider J, Kahnoski R et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res 2013; 73: 2044–2051.
Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012; 22: 66–79.
Itoh K . [Disease regulation by Nrf2 antioxidant system]. Seikagaku 2009; 81: 447–455.
Nguyen T, Nioi P, Pickett CB . The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009; 284: 13291–13295.
Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT . Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 2010; 13: 1713–1748.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475: 106–109.
Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 2011; 477: 225–228.
Shambaugh GE 3rd . Urea biosynthesis I. The urea cycle and relationships to the citric acid cycle. Am J Clin Nutr 1977; 30: 2083–2087.
Adam J, Yang M, Bauerschmidt C, Kitagawa M, O’Flaherty F, Maheswaran P et al. A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell reports 2013; S2211-1247: 00173–00173.
Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2012; 481: 385–388.
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012; 481: 380–384.
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA 2011; 108: 19611–19616.
Kaelin WG Jr . Synthetic lethality: a framework for the development of wiser cancer therapeutics. Genome Med 2009; 1: 99.
Rankin EB, Tomaszewski JE, Haase VH . Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 2006; 66: 2576–2583.
Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 2008; 14: 979–984.
Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011; 469: 539–542.
Copeland NG, Jenkins NA . Harnessing transposons for cancer gene discovery. Nat Rev Cancer 2010; 10: 696–706.
Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA . Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005; 436: 221–226.
Koglin N, Mueller A, Berndt M, Schmitt-Willich H, Toschi L, Stephens AW et al. Specific PET imaging of xC- transporter activity using a (1)(8)F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin Cancer Res 2011; 17: 6000–6011.
Qu W, Zha Z, Ploessl K, Lieberman BP, Zhu L, Wise DR et al. Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J Am Chem Soc 2011; 133: 1122–1133.
Acknowledgements
The Cancer Biology and Metabolism Group, led by Dr Pollard, is funded by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 310837 and Cancer Research UK. Professor Soga is funded from a Grant-in-Aid for scientific research on Innovative Areas, Japan No. 22134007 and the Yamagata Prefectural Government and City of Tsuruoka.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor Soga is a founder of Human Metabolome Technologies. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Adam, J., Yang, M., Soga, T. et al. Rare insights into cancer biology. Oncogene 33, 2547–2556 (2014). https://doi.org/10.1038/onc.2013.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.222
Keywords
This article is cited by
-
Links between mitochondrial retrograde response and mitophagy in pathogenic cell signalling
Cellular and Molecular Life Sciences (2021)
-
α-ketoglutarate dehydrogenase inhibition counteracts breast cancer-associated lung metastasis
Cell Death & Disease (2018)
-
Features and regulation of non-enzymatic post-translational modifications
Nature Chemical Biology (2018)
-
Metabolic implications of hypoxia and pseudohypoxia in pheochromocytoma and paraganglioma
Cell and Tissue Research (2018)
-
Targeting glycogen metabolism in bladder cancer
Nature Reviews Urology (2015)