Abstract
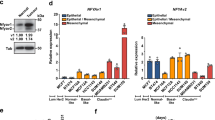
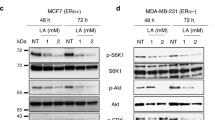
Upregulation of lipogenesis is a hallmark of cancer and blocking the lipogenic pathway is known to cause tumor cell death by apoptosis. However, the exact role of lipogenesis in tumor initiation is as yet poorly understood. We examined the expression profile of key lipogenic genes in clinical samples of ductal carcinoma in situ (DCIS) of breast cancer and found that these genes were significantly upregulated in DCIS. We also isolated cancer stem-like cells (CSCs) from DCIS.com cell line using cell surface markers (CS24−CD44+ESA+) and found that this cell population has significantly higher tumor-initiating ability to generate DCIS compared with the non-stem-like population. Furthermore, the CSCs showed significantly higher level of expression of all lipogenic genes than the counterpart population from non-tumorigenic breast cancer cell line, MCF10A. Importantly, ectopic expression of SREBP1, the master regulator of lipogenic genes, in MCF10A significantly enhanced lipogenesis in stem-like cells and promoted cell growth as well as mammosphere formation. Moreover, SREBP1 expression significantly increased the ability of cell survival of CSCs from MCF10AT, another cell line that is capable of generating DCIS, in mouse and in cell culture. These results indicate that upregulation of lipogenesis is a pre-requisite for DCIS formation by endowing the ability of cell survival. We have also shown that resveratrol was capable of blocking the lipogenic gene expression in CSCs and significantly suppressed their ability to generate DCIS in animals, which provides us with a strong rationale to use this agent for chemoprevention against DCIS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Barnes NL, Ooi JL, Yarnold JR, Bundred NJ . Ductal carcinoma in situ of the breast. BMJ 2012; 344: e797.
Porter DA, Krop IE, Nasser S, Sgroi D, Kaelin CM, Marks JR et al. A SAGE (serial analysis of gene expression) view of breast tumor progression. Cancer Res 61: 5697–5702 2001.
Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA 2003; 100: 5974–5979.
Menendez JA, Lupu R . Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007; 7: 763–777.
Swinnen JV, Brusselmans K, Verhoeven G . Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 2006; 9: 358–365.
Horton JD, Goldstein JL, Brown MS . SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131.
Rashid A, Pizer ES, Moga M et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol 1997; 150: 201–208.
Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP . Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res 1997; 3: 2115–2120.
Pizer ES, Chrest FJ, Di Giuseppe JA, Han WF . Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res 1998; 58: 4611–4615.
Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res 2006; 66: 5934–5940.
Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat 2011; 130: 387–398.
Pandey PR, Liu W, Xing F, Fukuda K, Watabe K . Anti-cancer drugs targeting fatty acid synthase (FAS). Recent Pat Anticancer Drug Discov 2012; 7: 185–197.
Liu W, Furuta E, Shindo K, Watabe M, Xing F, Pandey PR et al. Cacalol, a natural sesquiterpene, induces apoptosis in breast cancer cells by modulating Akt-SREBP-FAS signaling pathway. Breast Cancer Res Treat 2011; 128: 57–68.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Heppner GH, Wolman SR . MCF-10AT: a model for human breast cancer development. Breast J 1999; 5: 122–129.
Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR . MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol 1996; 148: 313–319.
Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ et al. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst 1993; 85: 1725–1732.
Shimano H . Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res 2001; 40: 439–452.
Brown MS, Goldstein JL . The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997; 89: 331–340.
Yamashita T, Honda M, Takatori H, Nishino R, Minato H, Takamura H et al. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol 2009; 50: 100–110.
Huang WC, Li X, Liu J, Lin J, Chung LW . Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res 2012; 10: 133–142.
Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 2008; 68: 1003–1011.
Damonte P, Hodgson JG, Chen JQ, Young LJ, Cardiff RD, Borowsky AD . Mammary carcinoma behavior is programmed in the precancer stem cell. Breast Cancer Res 2008; 10: R50.
Menendez JA, Lupu R . Oncogenic properties of the endogenous fatty acid metabolism: Molecular pathology of fatty acid synthase in cancer cells. Curr Opin Clin Nutr Metab Care 2006; 9: 346–357.
Esslimani-Sahla M, Thezenas S, Siminy-Lafontaine J, Kramar A, Lavaill R, Chalbos D et al. Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int J Cancer 2007; 120: 224–229.
Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC et al. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol 2000; 31: 1068–1073.
Baron A, Migita T, Tang D, Loda M . Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem 2004; 91: 47–53.
Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 2009; 101: 519–532.
Alberola-Ila J, Hernandez-Hoyos G . The Ras/MAPK cascade and the control of positive selection. Immunol Rev 2003; 191: 79–96.
Ramjaun AR, Downward J . Ras and phosphoinositide 3-kinase: partners in development and tumorigenesis. Cell Cycle 2007; 6: 2902–2905.
McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006; 46: 249–279.
Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI (3) K and PKB. Nature 1997; 385: 544–548.
Carón RW, Yacoub A, Mitchell C, Zhu X, Hong Y, Sasazuki T et al. Radiation-stimulated ERK1/2 and JNK1/2 signaling can promote cell cycle progression in human colon cancer cells. Cell Cycle 2005; 4: 456–464.
Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA . Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell prolif 2008; 41: 59–85.
Valverde AM, Navarro P, Benito M, Lorenzo M . H-ras induces glucose uptake in brown adipocytes in an insulin- and phosphatidylinositol 3-kinase-independent manner. Exp Cell Res 1998; 243: 274–281.
Guillet-Deniau I, Pichard AL, Koné A, Esnous C, Nieruchalski M, Girard J et al. Glucose induces de novo lipogenesis in rat muscle satellite cells through a sterol-regulatory-element-binding-protein-1c-dependent pathway. J Cell Sci 2004; 117: 1937–1944.
Uttarwar L, Gao B, Ingram AJ, Krepinsky JC . SREBP-1 activation by glucose mediates TGF-β upregulation in mesangial cells. Am J Physiol Renal Physiol 2012; 302: 329–341.
Janes PW, Daly RJ, deFazio A, Sutherland RL . Activation of the Ras signaling pathway in human breast cancer cells overexpressing erbB-2. Oncogene 1994; 9: 3601–3608.
Von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR . Ras activation in human breast cancer. Breast Cancer Res Treat 2000; 62: 51–62.
Lee CM, Shrieve DC, Zempolich KA, Lee RJ, Hammond E, Handrahan DL et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol 2005; 99: 415–421.
Chuthapisith S, Eremin J, El-Sheemey M, Eremin O . Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol 2010; 19: 27–32.
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, Van Buren G et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 2009; 69: 1951–1957.
Diehn M, Clarke MF . Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst 2006; 98: 1755–1757.
Gu G, Yuan J, Wills M, Kasper S . Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 2007; 67: 4807–4815.
Allan AL, Vantyghem SA, Tuck AB, Chambers AF . Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis 2007; 26: 87–98.
Okuda H, Kobayashi A, Xia B, Watabe M, Pai SK, Hirota S et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res 2012; 72: 537–547.
Acknowledgements
This work was supported by the National Institutes of Health (Grants R01CA124650, R01CA129000 and R01CA124650-04S1 to KW), the US Department of Defense (BC096982 to KW) and McElroy Foundation (to KW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Pandey, P., Xing, F., Sharma, S. et al. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene 32, 5111–5122 (2013). https://doi.org/10.1038/onc.2012.519
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.519
Keywords
This article is cited by
-
Metabolic Regulation: A Potential Strategy for Rescuing Stem Cell Senescence
Stem Cell Reviews and Reports (2022)
-
Metabolic convergence on lipogenesis in RAS, BCR-ABL, and MYC-driven lymphoid malignancies
Cancer & Metabolism (2021)
-
Classification of Homo sapiens gene behavior using linear discriminant analysis fused with minimum entropy mapping
Medical & Biological Engineering & Computing (2021)
-
The antimetastatic activity of orlistat is accompanied by an antitumoral immune response in mouse melanoma
Cancer Chemotherapy and Pharmacology (2020)
-
PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma
Medical Oncology (2015)