Abstract
Voltage-gated ion channels respond to transmembrane electric fields through reorientations of the positively charged S4 helix within the voltage-sensing domain (VSD). Despite a wealth of structural and functional data, the details of this conformational change remain controversial. Recent electrophysiological evidence showed that equilibrium between the resting ('down') and activated ('up') conformations of the KvAP VSD from Aeropyrum pernix can be biased through reconstitution in lipids with or without phosphate groups. We investigated the structural transition between these functional states, using site-directed spin-labeling and EPR spectroscopic methods. Solvent accessibility and interhelical distance determinations suggest that KvAP gates through S4 movements involving an ∼3-Å upward tilt and simultaneous ∼2-Å axial shift. This motion leads to large accessibly changes in the intracellular water-filled crevice and supports a new model of gating that combines structural rearrangements and electric-field remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9, 323–332 (2008).
Catterall, W.A. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron 67, 915–928 (2010).
Swartz, K.J. Sensing voltage across lipid membranes. Nature 456, 891–897 (2008).
Jiang, Y. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003).
Long, S.B., Tao, X., Campbell, E.B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Zhang, X. et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134 (2012).
Clayton, G.M., Altieri, S., Heginbotham, L., Unger, V.M. & Morais-Cabral, J.H. Structure of the transmembrane regions of a bacterial cyclic nucleotide-regulated channel. Proc. Natl. Acad. Sci. USA 105, 1511–1515 (2008).
Ramu, Y., Xu, Y. & Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699 (2006).
Schmidt, D., Jiang, Q.-X. & MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444, 775–779 (2006).
Xu, Y., Ramu, Y. & Lu, Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 451, 826–829 (2008).
Zheng, H., Liu, W., Anderson, L.Y. & Jiang, Q.-X. Lipid-dependent gating of a voltage-gated potassium channel. Nat. Commun. 2, 250 (2011).
McHaourab, H.S., Steed, P.R. & Kazmier, K. Toward the fourth dimension of membrane protein structure: insight into dynamics from spin-labeling EPR spectroscopy. Structure 19, 1549–1561 (2011).
Cuello, L.G., Cortes, D.M. & Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 (2004).
Chakrapani, S., Cuello, L.G., Cortes, D.M. & Perozo, E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure 16, 398–409 (2008).
Chakrapani, S., Sompornpisut, P., Intharathep, P., Roux, B. & Perozo, E. The activated state of a sodium channel voltage sensor in a membrane environment. Proc. Natl. Acad. Sci. USA 107, 5435–5440 (2010).
Krepkiy, D., Gawrisch, K. & Swartz, K.J. Structural interactions between lipids, water and S1–S4 voltage-sensing domains. J. Mol. Biol. 423, 632–647 (2012).
Yang, N. & Horn, R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 15, 213–218 (1995).
Ahern, C.A. & Horn, R. Focused electric field across the voltage sensor of potassium channels. Neuron 48, 25–29 (2005).
Asamoah, O.K., Wuskell, J.P., Loew, L.M. & Bezanilla, F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron 37, 85–97 (2003).
Krepkiy, D. et al. Structure and hydration of membranes embedded with voltage-sensing domains. Nature 462, 473–479 (2009).
Tombola, F., Pathak, M.M. & Isacoff, E.Y. How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 22, 23–52 (2006).
Yang, N., George, A.L. & Horn, R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122 (1996).
Larsson, H.P., Baker, O.S., Dhillon, D.S. & Isacoff, E.Y. Transmembrane movement of the shaker K+ channel S4. Neuron 16, 387–397 (1996).
Mannuzzu, L.M., Moronne, M.M. & Isacoff, E.Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271, 213–216 (1996).
Cha, A. & Bezanilla, F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 19, 1127–1140 (1997).
Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 63, 419–446 (2012).
Zou, P. & McHaourab, H.S. Increased sensitivity and extended range of distance measurements in spin-labeled membrane proteins: Q-band double electron-electron resonance and nanoscale bilayers. Biophys. J. 98, L18–L20 (2010).
Fleissner, M.R. et al. Structure and dynamics of a conformationally constrained nitroxide side chain and applications in EPR spectroscopy. Proc. Natl. Acad. Sci. USA 108, 16241–16246 (2011).
Rayes, R.F., Kálai, T., Hideg, K., Geeves, M.A. & Fajer, P.G. Dynamics of tropomyosin in muscle fibers as monitored by saturation transfer EPR of bi-functional probe. PLoS ONE 6, e21277 (2011).
Jiang, Y., Ruta, V., Chen, J., Lee, A. & MacKinnon, R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423, 42–48 (2003).
Lee, S.-Y., Lee, A., Chen, J. & MacKinnon, R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci. USA 102, 15441–15446 (2005).
Catterall, W.A. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 55, 953–985 (1986).
Guy, H.R. & Seetharamulu, P. Molecular model of the action potential sodium channel. Proc. Natl. Acad. Sci. USA 83, 508–512 (1986).
Ruta, V., Chen, J. & MacKinnon, R. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell 123, 463–475 (2005).
Chanda, B. & Bezanilla, F. A common pathway for charge transport through voltage-sensing domains. Neuron 57, 345–351 (2008).
Sompornpisut, P., Roux, B. & Perozo, E. Structural refinement of membrane proteins by restrained molecular dynamics and solvent accessibility data. Biophys. J. 95, 5349–5361 (2008).
Koag, M.-C. & Papazian, D.M. Voltage-dependent conformational changes of KVAP S4 segment in bacterial membrane environment. Channels (Austin) 3, 356–365 (2009).
Faure, É., Starek, G., McGuire, H., Bernèche, S. & Blunck, R. A limited 4 Å radial displacement of the S4–S5 linker is sufficient for internal gate closing in Kv channels. J. Biol. Chem. 287, 40091–40098 (2012).
Tao, X., Lee, A., Limapichat, W., Dougherty, D.A. & MacKinnon, R. A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010).
Lacroix, J.J. & Bezanilla, F. Control of a final gating charge transition by a hydrophobic residue in the S2 segment of a K+ channel voltage sensor. Proc. Natl. Acad. Sci. USA 108, 6444–6449 (2011).
Mchaourab, H.S., Lietzow, M.A., Hideg, K. & Hubbell, W.L. Motion of spin-labeled side chains in T4 lysozyme: correlation with protein structure and dynamics. Biochemistry 35, 7692–7704 (1996).
Altenbach, C., Greenhalgh, D.A., Khorana, H.G. & Hubbell, W.L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 91, 1667–1671 (1994).
Jeschke, G. Distance measurements in the nanometer range by pulse EPR. ChemPhysChem 3, 927–932 (2002).
Jeschke, G. et al. DeerAnalysis2006: a comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 30, 473–498 (2006).
Sompornpisut, P., Roux, B. & Perozo, E. Structural refinement of membrane proteins by restrained molecular dynamics and solvent accessibility data. Biophys. J. 95, 5349–5361 (2008).
Fleissner, M.R. et al. Structure and dynamics of a conformationally constrained nitroxide side chain and applications in EPR spectroscopy. Proc. Natl. Acad. Sci. USA 108, 16241–16246 (2011).
Acknowledgements
We are thankful to H. Mchaourab and R. Stein for generous sharing of the Q-band spectrometer, assistance in data gathering and general insightful comments. We thank G. Meyer for the scripts to analyze power-saturation data. We thank F. Bezanilla, B. Roux and members of the Perozo, Bezanilla and Roux laboratories for helpful discussions and invaluable experimental advice. This work was supported in part by US National Institutes of Health grants R01-GM57846 and U54-GM74946 (to E.P.) and Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University grant RES560530217-HR (to P.S.).
Author information
Authors and Affiliations
Contributions
Q.L. and E.P. designed the strategy, Q.L. and S.W. performed the experiments, P.S. performed the MD simulation, Q.L. analyzed the data, and E.P. and Q.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
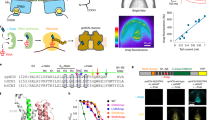
Supplementary Figure 1 Sample characterization.
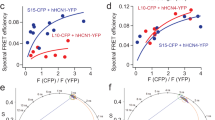
1. FRET assay indicates no aggregation of KvAP-VSD in both PC:PG and DOTAP liposomes. (a) Cartoon representation of FRET assay to evaluate the aggregation behavior. KvAP-VSD mutant A111C (b) was individually labeled with fluorescence donor and acceptor, then mixed at 1:1 molar ratio and reconstituted into liposomes. FRET signal in the range of 560-580 nm indicates of closeness of fluorephores in liposome, thus the degree of protein aggregation (see Methods for details). (c) Amplitude normalized FRET spectra of KvAP-VSD in liposomes with incremental DOTAP contents. The intensity within region 560-580 nm is essentially the same among all tested conditions, which suggest no severe aggregation of KvAP-VSD in both POPC:POPG and DOTAP liposomes. (d) Conformation dependent polarity change upon increment of DOTAP content shown by the wavelength shift of fluorecin emission (grey region, (c)). The sigmoidal fit agrees very well with the titration results monitored by spin labeling methods (Fig. 2). 2. DOTAP liposome is physically equivalent to POPC:POPG liposome regarding to oxygen and NiEdda accessibilities. (e) EPR spectra of nitroxide radicals at various depths in both PC:PG and DOTAP liposomes, probed by radical containing phosphocholine lipids (see Methods for details). (f) No NiEdda accessibility (ΠNi) was observed at all studied positions in both liposomes. (g) Oxygen accessibility (ΠO2) was centralized in the middle of lipid bilayer and decreased toward the surface of liposomes. Both liposomes have the same pattern with slightly different amplitudes.
Supplementary Figure 2 Data interpretation.
1. The tilt of S4 from PC:PG to DoTAP shown by the vector presentation ΠNiEdda. ΠNi in DOTAP was mapped onto crystal structure with linear blue scale. There is no water accessibility to the center of membrane. The net difference of ΠNi on extracellular and intracellular ends clearly indicates a tilt trend in the direction opposite to the ΠNi. 2. Coupling of sensor, pore and the ion conduction. KvAP G-V curves (c) with different non-phosphate lipid contents (50%, 67% and 80%) were simulated with published Vh (b) and z values. At 0 mV, the ion conduction decreases upon DOTAP content increment from 40% to 100% ((d) black square), which overlaps exactly with the synchronized mobility increase of both S4 bottom and pore. The coupling of sensor and the pore of the KvAP leads to the functional difference.
Supplementary Figure 3 Spin-labeling efficiency on KvAP-VSD cysteine mutant.
(a) ESI-TOF mass spectrometry analysis for labeled (with either MTSL or bifunctional spin label) KvAP VSD cysteine mutants (either 118C/121C or 121C). The m/z of ionized fragments (top) and deconvoluted mass (bottom) were shown for each of four samples. The spin labeled protein has predominant peak in the MS spectra. (b) Summary of the MS results confirming the correct bi-functional attachment of Bi-SL onto the sensor. In particular, there are three possibilities for a bifunctional spin label attaching to double cysteine mutant: 1. Sinlge Bi-SL on one of the cysteine (expected MW, 16954.7); 2. Two Bi-SL on both single cysteine (expected MW, 17262.8); 3. Single Bi-SL on two cysteine (expected MW, 16874.5). The MS unambiguously confirms that both reaction group of Bi-SL were removed through reaction with two cysteine groups. (c) Continuous EPR spectra of MTSL labeled (left) and Bi-SL labeled (right) samples. Two MTSL on to adjacent positions shows characteristic dipolar coupling (left, red). At the labeling condition with 20X molar excess of spin label, if a Bi-SL didn't react with both cysteines together, the double cysteine mutant would be labeled with two Bi-SL which will show significant bipolar coupling feature in CW-EPR spectra.
Supplementary Figure 4 Background-corrected echo decay and distance distribution of ten pairs of DEER measurements in PCPG and DOTAP liposomes.
(a) The dipolar evolution in PCPG (grey circle) and DOTAP (blue circle) were analyzed by Tikhonov regularization. The fit and the resulting distance distribution are shown in red (PCPG) and black (DOTAP) lines. The distances are consistent with geometric patterns indicated by crystal structure. Predominant single main peak (above the grey region) is clearly resolved for all 10 pairs of distances in two liposomes ((b) PCPG and (c) DOTAP) among the range of 22 to 38 Å, which lay in the most sensitive region of the pulsed EPR method. All 10 distances measured with 118/121 (left, solid line) are larger than the corresponding distances with 121/125 (left, dash line), which are consistent with 118/121 are further than 121/125 to all reference points (d). The same consistency was also observed for 40/44 > 39/43 ((d), top) and 72/75 > 74/77 ((d), bottom) in all measured distances ((b) and (c)).
Supplementary Figure 5 General scheme of MD simulation and the structural models.
(a) Simple representation of the pseudo-spin (EP1, EP2 and EP3) exposure to different environments in a membrane protein. (b) schematic representation of a protein attached with the HO-1944 cross-linked nitroxide spin label which transforms into a simple pseudoatom (EPX) representation. EPX is covalently attached to the backbone Cαi and Cαi +3 (or Cαi +4). (c) The ten EPX-EPX distance restraints used for restrained molecular dynamics calculation. (d) During the RMD run, distance and accessibility (PaDSAR method) restraints were imposed. (e) Superimposition of crystal structure (PDB 1ORS) (yellow) and the simulated Up state model (blue). (f) Top: the best ten structures of Down state (red) models was superimposed with Up state model (blue). Bottom: structure comparison between the two average models representing the up (blue) and down (red) conformations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 3998 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Wanderling, S., Sompornpisut, P. et al. Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain. Nat Struct Mol Biol 21, 160–166 (2014). https://doi.org/10.1038/nsmb.2747
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2747
This article is cited by
-
Quaternary structure independent folding of voltage-gated ion channel pore domain subunits
Nature Structural & Molecular Biology (2022)
-
Expression and Purification of the Pain Receptor TRPV1 for Spectroscopic Analysis
Scientific Reports (2017)
-
Disulfide mapping the voltage-sensing mechanism of a voltage-dependent potassium channel
Scientific Reports (2016)
-
Structure of an E. coli integral membrane sulfurtransferase and its structural transition upon SCN− binding defined by EPR-based hybrid method
Scientific Reports (2016)
-
Structural basis for the inhibition of voltage-dependent K+ channel by gating modifier toxin
Scientific Reports (2015)