Abstract
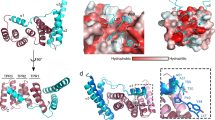
SecB is a bacterial chaperone involved in directing pre-protein to the translocation pathway by its specific interaction with the peripheral membrane ATPase SecA. The SecB-binding site on SecA is located at its C terminus and consists of a stretch of highly conserved residues. The crystal structure of SecB in complex with the C-terminal 27 amino acids of SecA from Haemophilus influenzae shows that the SecA peptide is structured as a CCCH zinc-binding motif. One SecB tetramer is bound by two SecA peptides, and the interface involves primarily salt bridges and hydrogen bonding interactions. The structure explains the importance of the zinc-binding motif and conserved residues at the C terminus of SecA in its high-affinity binding with SecB. It also suggests a model of SecB-SecA interaction and its implication for the mechanism of pre-protein transfer in bacterial protein translocation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fekkes, P. & Driessen, A.J. Protein targeting to the bacterial cytoplasmic membrane. Microbiol. Mol. Biol. Rev. 63, 161–173 (1999).
Driessen, A.J., Manting, E.H. & van der Does, C. The structural basis of protein targeting and translocation in bacteria. Nat. Struct. Biol. 8, 492–498 (2001).
Kim, J. & Kendall, D.A. Sec-dependent protein export and the involvement of the molecular chaperone SecB. Cell Stress Chaperones 5, 267–275 (2000).
Kumamoto, C.A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl. Acad. Sci. USA 86, 5320–5324 (1989).
Randall, L.L. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science 257, 241–245 (1992).
Fekkes, P. et al. Preprotein transfer to the Escherichia coli translocase requires the co-operative binding of SecB and the signal sequence to SecA. Mol. Microbiol. 29, 1179–1190 (1998).
Fekkes, P., van der Does, C. & Driessen, A.J. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 16, 6105–6113 (1997).
Economou, A. & Wickner, W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78, 835–843 (1994).
Miller, A., Wang, L. & Kendall, D.A. SecB modulates the nucleotide-bound state of SecA and stimulates ATPase activity. Biochemistry 41, 5325–5332 (2002).
Woodbury, R.L. et al. Complexes between protein export chaperone SecB and SecA. Evidence for separate sites on SecA providing binding energy and regulatory interactions. J. Biol. Chem. 275, 24191–24198 (2000).
Fekkes, P., de Wit, J.G., Boorsma, A., Friesen, R.H. & Driessen, A.J. Zinc stabilizes the SecB binding site of SecA. Biochemistry 38, 5111–5116 (1999).
Driessen, A.J., Fekkes, P. & van der Wolk, J.P. The Sec system. Curr. Opin. Microbiol. 1, 216–222 (1998).
Kimsey, H.H., Dagarag, M.D. & Kumamoto, C.A. Diverse effects of mutation on the activity of the Escherichia coli export chaperone SecB. J. Biol. Chem. 270, 22831–22835 (1995).
Xu, Z., Knafels, J.D. & Yoshino, K. Crystal structure of the bacterial protein export chaperone SecB. Nat. Struct. Biol. 7, 1172–1177 (2000).
Hunt, J.F. et al. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 297, 2018–2026 (2002).
Sharma, V. et al. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl. Acad. Sci. USA 100, 2243–2248 (2003).
Rajapandi, T. & Oliver, D. Carboxy-terminal region of Escherichia coli SecA ATPase is important to promote its protein translocation activity in vivo. Biochem. Biophys. Res. Commun. 200, 1477–1183 (1994).
Harding, M.M. Geometry of metal-ligand interactions in proteins. Acta Crystallogr. D 57, 401–411 (2001).
Laity, J.H., Lee, B.M. & Wright, P.E. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 11, 39–46 (2001).
Mackereth, C.D., Arrowsmith, C.H., Edwards, A.M. & McIntosh, L.P. Zinc-bundle structure of the essential RNA polymerase subunit RPB10 from Methanobacterium thermoautotrophicum. Proc. Natl. Acad. Sci. USA 97, 6316–6321 (2000).
Evans, J.C. et al. Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure 10, 1159–1171 (2002).
Zhou, Z.S., Peariso, K., Penner-Hahn, J.E. & Matthews, R.G. Identification of the zinc ligands in cobalamin-independent methionine synthase (MetE) from Escherichia coli. Biochemistry 38, 15915–15926 (1999).
Fleischmann, R.D. et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269, 496–512 (1995).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Acknowledgements
We thank J. Stuckey for maintaining the X-ray facility at the University of Michigan Medical School, K. Yoshino for making the GST fusion construct, D. Peisach for help in data collection and crystallography, K. Brister for access and help at the Advanced Photon Source BioCARs beamline 14-BM-C, and C. Fierke and R. Matthews for critically reading the manuscript. This work was supported by a US National Institutes of Health grant to Z.X. and the University of Michigan Biological Scholar Program. Z.X. is a Pew scholar in Biomedical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zhou, J., Xu, Z. Structural determinants of SecB recognition by SecA in bacterial protein translocation. Nat Struct Mol Biol 10, 942–947 (2003). https://doi.org/10.1038/nsb980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb980