Key Points
-
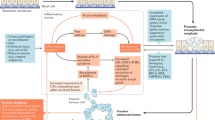
Epidemiological data suggest a causal link between benign prostatic hyperplasia (BPH) and prostatic inflammation
-
Stimuli including infectious agents, urinary reflux, metabolic syndrome, the ageing process and autoimmune responses can trigger dysregulation of the prostate immune system through several molecular pathways involving the development of inflammatory infiltrates
-
Tissue damage and chronic tissue healing could result in the development of BPH nodules
-
Several drugs targeting prostatic inflammation have been tested for the management of BPH and its symptoms, but concerns regarding their efficacy and safety limit their use in clinical practice
-
Targeting prostate inflammation could offer a target for new treatment strategies in patients with BPH
Abstract
Benign prostatic hyperplasia (BPH) is the most common urological disease in elderly men. Epidemiological data suggest a causal link between this condition and prostatic inflammation. The prostate is an immune-competent organ characterized by the presence of a complex immune system. Several stimuli, including infectious agents, urinary reflux, metabolic syndrome, the ageing process, and autoimmune response, have been described as triggers for the dysregulation of the prostatic immune system via different molecular pathways involving the development of inflammatory infiltrates. From a pathophysiological standpoint, subsequent tissue damage and chronic tissue healing could result in the development of BPH nodules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kramer, G., Mitteregger, D. & Marberger, M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur. Urol. 51, 1202–1216 (2007).
Robert, G., Descazeaud, A., Allory, Y., Vacherot, F. & de la Taille, A. Should we investigate prostatic inflammation for the management of benign prostatic hyperplasia? Eur. Urol. Suppl. 8, 879–886 (2009).
Sciarra, A. et al. Inflammation and chronic prostatic diseases: evidence for a link? Eur. Urol. 52, 964–972 (2007).
De Nunzio, C., Albisinni, S., Gacci, M. & Tubaro, A. The role of inflammation in the progression of benign prostatic hyperplasia. Curr. Bladder Dysfunct. Rep. 8, 142–149 (2013).
Tubaro, A., De Nunzio, C., Puccini, F. & Presicce, F. The evolving picture of lower urinary tract symptom management. Eur. Urol. 67, 271–272 (2015).
Soler, R. et al. Futute direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur. Urol. 64, 610–621 (2013).
De Nunzio, C. Aronson, W., Freedland, S. J., Giovannucci, E. & Parsons, J. K. The correlation between metabolic syndrome and prostatic diseases. Eur. Urol. 61, 560–570 (2012).
Gacci, M. et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 16, 101–106 (2013).
Moore, D. Inflammation of the prostate gland. J. Urol. 38, 173–182 (1937).
Kramer, G. et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 52, 43–58 (2002).
Kramer, G. & Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 16, 25–29 (2006).
Steiner, G. E. et al. Expression and function of proinflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 56, 171–182 (2003).
Steiner, G. E. et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab. Invest. 83, 1131–1146 (2003).
Nickel, J. C. et al. Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J. Urol. 178, 896–900 (2007).
Nickel, J. C. et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur. Urol. 54, 1379–1384 (2008).
Roehrborn, C. G. et al. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPH: results from the MTOPS study. J. Urol. 173, 346 (2005).
Shortliffe, L. M., Werner, N. & Stamey, T. A. The detection of a local prostatic immunologic response to bacterial prostatitis. J. Urol. 125, 509–515 (1981).
Vykhovanets, E. V., Resnick, M. I. & Marengo, S. R. The healthy rat prostate contains high levels of natural killer-like cells and unique subsets of CD4+ helper-inducer T cells: implications for prostatitis. J. Urol. 173, 1004–1010 (2005).
Di Carlo, E., Magnasco, S., D'Antuono, T., Tenaglia, R. & Sorrentino, C. The prostate-associated lymphoid tissue (PALT) is linked to the expression of homing chemokines CXCL13 and CCL21. Prostate 67, 1070–1080 (2007).
De Nunzio, C. et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur. Urol. 60, 106–117 (2011).
Robert, G. et al. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate 69, 1774–1780 (2009).
Penna, G. et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J. Immunol. 182, 4056–4064 (2009).
Fibbi, B., Penna, G., Morelli, A., Adorini, L. & Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 33, 475–488 (2010).
Kramer, G. et al. Loss of CD38 correlates with simultaneous upregulation of human leukocyte antigen-DR in benign prostatic glands, but not in fetal or androgen-ablated glands, and is strongly related to gland atrophy. BJU Int. 91, 409–416 (2003).
Beadling, C. & Slifka, M. K. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Ther. Exp. (Warsz.) 54, 15–24 (2006).
Sfanos, K. S., Isaacs, W. B. & De Marzo, A. M. Infections and inflammation in prostate cancer. Am. J. Clin. Exp. Urol. 1, 3–11 (2013).
Elkahwaji, J. E. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res. Rep. Urol. 31, 1–10 (2012).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Nakai, Y., Nelson, W. G. & De Marzo, A. M. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 67, 1378–1384 (2007).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7, 256–269 (2007).
Bardan, R., Dumache, R., Dema, A., Cumpanas, A. & Bucuras, V. The role of prostatic inflammation biomarkers in the diagnosis of prostate diseases. Clin. Biochem. 47, 909–915 (2014).
Chokkalingam, A. P. et al. Prostate carcinoma risk subsquent to diagnosis of benign prostatic hyperplasia: a population-based cohort study in Sweden. Cancer 98, 1727–1734 (2003).
Ørsted, D. D. & Bojesen, S. E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 10, 49–54 (2013).
Ørsted, D. D., Bojesen, S. E., Nielsen, S. F. & Nordestgaard, B. G. Association of clinical benign prostate hyperplasia with prostate cancer incidence and mortality revisited: a nationwide cohort study of 3,009,258 men. Eur. Urol. 60, 691–698 (2011).
Vignozzi, L. & Maggi, M. Intriguing data on inflammation and prostate cancer. Nat. Rev. Urol. 11, 369–370 (2014).
Roehrborn, C. G. Definition of at-risk patients: baseline variables. BJU Int. 97, 7–11 (2006).
Gandaglia, G. et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 4, 432–441 (2013).
Zlotta, A. R. et al. Prevalence of inflammation and benign prostatic hyperplasia on autopsy in Asian and Caucasian men. Eur. Urol. 66, 619–622 (2014).
He, Q. et al. Metabolic syndrome, inflammation and lower urinary tract symptoms: possible translational links. Prostate Cancer Prostatic Dis. 19, 7–13 (2016).
Kupelian, V. et al. Association of overactive bladder and C-reactive protein levels. Results from the Boston Area Community Health (BACH) Survey. BJU Int. 110, 401–407 (2012).
De Nunzio, C. et al. Metabolic syndrome and lower urinary tract symptoms in patients with benign prostatic enlargement: a possible link to storage symptoms. Urology 84, 1181–1187 (2014).
Konig, J. E., Senge, T., Allhoff, E. P. & Konig, W. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate 58, 121–129 (2004).
McKenzie, B. S., Kastelein, R. A. & Cua, D. J. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 27, 17–23 (2006).
Wang, W., Bergh, A. & Damber, J. E. Chronic inflammation in benign prostatic hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 61, 60–72 (2004).
De Angulo, A., Faris, R., Daniel, B., Jolly, C. & de Graffenried, L. Age-related increase in IL-17 activates pro-inflammatory signaling in prostate cells. Prostate 75, 49–62 (2015).
Descazeaud, A. et al. Transforming growth factor β-receptor II protein expression in benign prostatic hyperplasia is associated with prostate volume and inflammation. BJU Int. 108, E23–E28 (2011).
Funahashi, Y. et al. Upregulation of androgen-responsive genes and transforming growth factor-β1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate 74, 337–345 (2014).
Funahashi, Y. et al. Influence of E. coli-induced prostatic inflammation on expression of androgen-responsive genes and transforming growth factor beta 1 cascade genes in rats. Prostate 75, 381–389 (2015).
Izumi, K., Mizokami, A., Lin, W. J., Lai, K. P. & Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 182, 1942–1949 (2013).
Kim, H. J. et al. Pathogenic role of HIF-1α in prostate hyperplasia in the presence of chronic inflammation. Biochim. Biophys. Acta 1832, 183–194 (2013).
Wang, X. et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J. Biol. Chem. 287, 18376–18385 (2012).
Hamakawa, T. et al. Interleukin-18 may lead to benign prostatic hyperplasia via thrombospondin-1 production in prostatic smooth muscle cells. Prostate 74, 590–601 (2014).
Kashyap, M. et al. Inflammasomes are important mediators of prostatic inflammation associated with BPH. J. Inflamm (Lond.) 17, 12–37 (2015).
Castro, P., Xia, C., Gomez, L., Lamb, D. J. & Ittmann, M. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate 60, 153–159 (2004).
Schauer, I. G., Ressler, S. J., Tuxhorn, J. A., Dang, T. D. & Rowley, D. R. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology 72, 205–213 (2008).
Giri, D. & Ittmann, M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am. J. Pathol. 157, 249–255 (2000).
Fujita, K. et al. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate 70, 473–481 (2010).
Wang, L., Yang, J. R., Yang, L. Y. & Liu, Z. T. Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med. Hypotheses 70, 1021–1023 (2008).
Briganti, A. et al. Benign prostatic hyperplasia and its aetiologies. Eur. Urol. Suppl. 8, 865–871 (2009).
Mosli, H. A. et al. Local inflammation influences oestrogen metabolism in prostatic tissue. BJU Int. 110, 274–282 (2012).
Monti, S. et al. Androgen concentrations and their receptors in the periurethral region are higher than those of the subcapsular zone in benign prostatic hyperplasia (BPH). J. Androl. 19, 428–433 (1998).
Nicholson, T. M., Sehgal, P. D., Drew, S. A., Huang, W. & Ricke, W. A. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation 85, 140–149 (2013).
Wu, Z. L., Yuan, Y., Geng, H. & Xia, S. J. Influence of immune inflammation on androgen receptor expression in benign prostatic hyperplasia tissue. Asian J. Androl. 14, 316–319 (2012).
Vignozzi, L. et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate 73, 789–800 (2013).
Vignozzi, L., Gacci, M. & Maggi, M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat. Rev. Urol. 13, 108–119 (2016).
Shankar, E. et al. High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-κB. Prostate 72, 233–243 (2012).
Tuncel, A. et al. Do prostatic infarction, prostatic inflammation and prostate morphology play a role in acute urinary retention? Eur. Urol. 48, 277–284 (2005).
Mishra, V. C. et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 100, 327–331 (2007).
van Vuuren, S. P., Heyns, C. F. & Zarrabi, A. D. Significance of histological prostatitis in patients with urinary retention and underlying benign prostatic hyperplasia or adenocarcinoma of the prostate. BJU Int. 109, 1194–1197 (2012).
Torkko, K. C. et al. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS Study. J. Urol. 194, 454–461 (2015).
Kwon, Y. K. et al. The effect of intraprostatic chronic inflammation on benign prostatic hyperplasia treatment. Kor. J. Urol. 51, 266–270 (2010).
Lee, H. N., Kim, T. H., Lee, S. J., Cho, W. Y. & Shim, B. S. Effects of prostatic inflammation on LUTS and alpha blocker treatment outcomes. Int. Braz. J. Urol. 40, 356–366 (2014).
Ge, R. et al. DNA methyl transferase 1 reduces expression of SRD5A2 in the aging adult prostate. Am. J. Pathol. 185, 870–882 (2015).
Lin-Tsai, O. et al. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate 74, 669–679 (2014).
Fan, Y. et al. Low intraprostatic DHT promotes the infiltration of CD8+ T cells in BPH tissues via modulation of CCL5 secretion. Mediators Inflamm. 2014, 397815 (2014).
Vignozzi, L. et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J. Endocrinol. 214, 31–43 (2012).
Tsujimura, A. et al. Histologic evaluation of human benign prostatic hyperplasia treated by dutasteride: a study by xenograft model with improved severe combined immunodeficient mice. Urology 85, 1–8 (2015).
Meigs, J. B., Mohr, B., Barry, M. J., Collins, M. M. & McKinlay, J. B. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J. Clin. Epidemiol. 54, 935–944 (2001).
Sutcliffe, S. et al. Non-steroidal anti-inflammatory drug use and the risk of benign prostatic hyperplasia-related outcomes and nocturia in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. BJU Int. 110, 1050–1059 (2012).
Kang, D. et al. Risk behaviours and benign prostatic hyperplasia. BJU Int. 93, 1241–1245 (2004).
St Sauver, J. L., Jacobson, D. J., McGree, M. E., Lieber, M. M. & Jacobsen, S. J. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am. J. Epidemiol. 164, 760–768 (2006).
Altavilla, D. et al. Effects of flavocoxid, a dual inhibitor of COX and 5 lipoxygenase enzymes, on benign prostatic hyperplasia. Br. J. Pharmacol. 167, 95–108 (2012).
Kahokehr, A., Vather, R., Nixon, A. & Hill, A. G. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. BJU Int. 111, 304–311 (2013).
Fourcade, R. O., Théret, N. & Taïeb, C. Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int. 101, 1111–1118 (2008).
Vela Navarrete, R., Garcia Cardoso, J. V., Barat, A., Manzarbeitia, F. & López Farré, A. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur. Urol. 44, 549–555 (2003).
Sirab, N. et al. Lipidosterolic extract of serenoa repens modulates the expression of inflammation related-genes in benign prostatic hyperplasia epithelial and stromal cells. Int. J. Mol. Sci. 14, 14301–14320 (2013).
Latil, A., Pétrissans, M. T., Rouquet, J., Robert, G. & de la Taille, A. Effects of hexanic extract of serenoa repens (permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 75, 1857–1867 (2015).
Vignozzi, L. et al. PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 73, 1391–1402 (2013).
Adorini, L. et al. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol). J. Steroid Biochem. Mol. Biol. 103, 689–693 (2007).
Parsons, J. K. & Kashefi, C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur. Urol. 53, 1228–1235 (2008).
Parsons, J. K. et al. Obesity increases and physical activity decreases lower urinary tract symptom risk in older men: the Osteoporotic Fractures in Men study. Eur. Urol. 60, 1173–1180 (2011).
De Nunzio, C. et al. 541 Metabolic abnormalities linked to an increased cardiovascular risk are associated with higher storage lower urinary tract symptoms. Eur. Urol. Suppl. 15, e541 (2016).
Tajik, N. et al. Effect of diet-induced weight loss on inflammatory cytokines in obese women. J. Endocrinol. Invest. 36, 211–215 (2013).
Khoo, J., Piantadosi, C., Worthley, S. & Wittert, G. A. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int. J. Obes. (Lond.) 34, 1396–1403 (2010).
Kristal, A. R. et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am. J. Epidemiol. 167, 925–934 (2008).
Tavani, A. et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur. Urol. 50, 549–554 (2006).
Rohrmann, S. et al. Association between serum concentrations of micronutrients and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey. Urology 64, 504–509 (2004).
Rohrmann, S., Giovannucci, E., Willett, W. C. & Platz, E. A. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am. J. Clin. Nutr. 85, 523–529 (2007).
Suzuki, S. et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am. J. Clin. Nutr. 75, 689–697 (2002).
De Nunzio, C. et al. Patients with prostatic inflammation undergoing transurethral prostatic resection have a larger early improvement of storage symptoms. Urology 86, 359–365 (2015).
Fujita, K. et al. White blood cell count is positively associated with benign prostatic hyperplasia. Int. J. Urol. 21, 308–312 (2014).
Menschikowski, M. et al. Serum levels of secreted group IIA phospholipase A2 in benign prostatic hyperplasia and prostate cancer: a biomarker for inflammation or neoplasia? Inflammation 35, 1113–1118 (2012).
Robert, G. et al. Biomarkers for the diagnosis of prostatic inflammation in benign prostatic hyperplasia. Prostate 71, 1701–1709 (2011).
Lotti, F. et al. Ultrasonographic and clinical correlates of seminal plasma interleukin-8 levels in patients attending an andrology clinic for infertility. Int. J. Androl 34, 600–613 (2011).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to researching data for this article, discussions of content, writing the article and editing and/or reviewing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol 13, 613–626 (2016). https://doi.org/10.1038/nrurol.2016.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.168
This article is cited by
-
Primary bladder neck obstruction in men—new perspectives in physiopathology
Prostate Cancer and Prostatic Diseases (2024)
-
Features of patients referring to the outpatient office due to benign prostatic hyperplasia: analysis of a national prospective cohort of 5815 cases
Prostate Cancer and Prostatic Diseases (2023)
-
Shuangshi Tonglin Capsule treats benign prostatic hyperplasia through the ROS/NLRP3 signaling pathway
International Urology and Nephrology (2023)
-
Comparative RNA-sequencing analysis of the prostate in a mouse model of benign prostatic hyperplasia with bladder outlet obstruction
Molecular and Cellular Biochemistry (2023)
-
Prostate luminal progenitor cells: from mouse to human, from health to disease
Nature Reviews Urology (2022)