Key Points
-
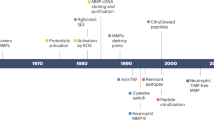
Alarmins are endogenous molecules that are rapidly released to the extracellular milieu during infection and tissue damage, activating receptors such as Toll-like receptors and receptor for advanced glycosylation end products
-
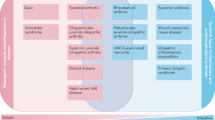
In arthritis, including degenerative joint disease (osteoarthritis (OA)) and chronic inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis and spondylarthropathy), extracellular levels of alarmins are associated with disease activity and joint destruction
-
In OA, S100 proteins and high mobility group protein B1 (HMGB1) are important alarmins that induce positive-feedback loops of synovial cell reactivation, inflammation and cartilage degradation
-
Alarmins involved in chronic inflammatory arthritis include S100 proteins, HMGB1, heat-shock proteins and IL-33; these alarmins represent important links between the innate and adaptive immune systems
-
The level of alarmins in serum and synovial fluid could provide useful diagnostic and prognostic biomarkers of arthritis, and inhibiting alarmin pathways could be a therapeutically beneficial approach
Abstract
Alarmins (also known as danger signals) are endogenous molecules that are released to the extracellular milieu after infection or tissue damage. Extracellular alarmins interact with specific receptors expressed by cells that are engaged in host defence to stimulate signalling pathways that result in initiation of innate and adaptive immune responses, triggering inflammation or tissue repair. Alarmins are considered to be markers of destructive processes that occur in degenerative joint diseases (primarily osteoarthritis (OA)) and chronic inflammatory joint diseases (such as rheumatoid arthritis, psoriatic arthritis and spondylarthropathy). In OA, high mobility group protein B1 (HMGB1) and S100 proteins, along with many other alarmins, are abundantly secreted by joint cells, promoting cartilage matrix catabolism, osteophyte formation, angiogenesis and hypertrophic differentiation. The involvement of alarmins in chronic inflammatory arthritides is suggested by their presence in serum at high levels in these conditions, and their expression within inflamed synovia and synovial fluid. S100 proteins, HMGB1, IL-33 and other endogenous molecules have deleterious effects on joints, and can recruit immune cells such as dendritic cells to inflamed synovia, initiating the adaptive immune response and perpetuating disease. Improving our understanding of the pathological mechanisms associated with these danger signals is important to enable the targeting of new therapeutic approaches for arthritis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Palazzo, C. et al. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 59, 134–138 (2016).
Abramson, S. B. & Attur, M. Developments in the scientific understanding of osteoarthritis. Arthritis. Res. Ther. 11, 227 (2009).
Bijlsma, J. W. et al. Osteoarthritis: an update with relevance for clinical practice. Lancet. 377, 2115–2126 (2011).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Foell, D. Wittkowski, H. & Roth, J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 7, 382–390 (2007).
Oppenheim, J. J. & Yang, D. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17, 359–365 (2005).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Houard, X., Goldring, M. B. & Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15, 375 (2013).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Harris, H. E. & Raucci, A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 7, 774–778 (2006).
Pugin, J. Dear SIRS, the concept of “alarmins” makes a lot of sense! Intensive Care Med. 34, 218–221 (2008).
Pisetsky, D. S. et al. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid. Redox. Signal. 15, 2209–2219 (2011).
Loeser, R. F. et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Chan, J. K. et al. Alarmins: awaiting a clinical response. J. Clin. Invest. 122, 2711–2719 (2012).
Manfredi, A. A. et al. Regulation of dendritic- and T-cell fate by injury-associated endogenous signals. Crit. Rev. Immunol. 29, 69–86 (2009).
Tamaki, Y. et al. Expression of Toll-like receptors and their signaling pathways in rheumatoid synovitis. J. Rheumatol. 38, 810–820 (2011).
Dumitriu, I. E. et al. Release of high mobility group BOX 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 174, 7506–7515 (2005).
Yang, D. et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 81, 59–66 (2007).
Yang, D. et al. The alarmin functions of high-mobility group proteins. Biochim. Biophys. Acta 1799, 157–163 (2010).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Denise, L. C. et al. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J. Immunol. 175, 8296–8302 (2005).
Seol, D. et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 64, 3626–3637 (2012).
Buckwalter, J. A. et al. The roles of mechanical stresses in the pathogenesis of osteoarthritis: implications for treatment of joint injuries. Cartilage 4, 286–294 (2013).
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Weinans, H. et al. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone 51, 190–196 (2012).
Sanchez, C. et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 64, 1193–1203 (2012).
Bidwell, J. P. et al. Is HMGB1 an osteocyte alarmin? J. Cell. Biochem. 103, 1671–1680 (2008).
Ke, X. et al. Synovial fluid HMGB-1 levels are associated with osteoarthritis severity. Clin. Lab. 61, 809–818 (2015).
Wang, L. C. et al. S100A12 levels in synovial fluid may reflect clinical severity in patients with primary knee osteoarthritis. Biomarkers 18, 216–220 (2013).
Wei, M. et al. Increased thymosin β4 levels in the serum and SF of knee osteoarthritis patients correlate with disease severity. Regul. Pept. 185, 34–36 (2013).
Denoble, A. E. et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. U.S.A. 108, 2088–2093 (2011).
Ayral, X. et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis: results of 1 year longitudinal arthroscopic studi in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Benito, M. J. et al. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Da, R. R. et al. B cell clonal expansion and somatic with osteoarthritis genes in the synovial membrane of patients hypermutation of Ig variable heavy chain. J. Immunol. 178, 557–565 (2007).
Sunahori, K. et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 8, 69 (2006).
Daghestani, H. N. et al.Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage 23, 1890–1896 (2015).
Van Lent, P. L. et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 64, 1466–1476 (2012).
Liu-Bryan, R. & Terkeltaub, R. T. The growing array of innate inflammatory ignition switches in osteoarthritis. Arthritis Rheum. 64, 2055–2058 (2012).
Marenholz, I. et al. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 322, 1111–1122 (2004).
Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 60, 540–551 (2003).
Ruse, M. et al. S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochem. 40, 3167–3173 (2001).
Meijer, B., Gearry, R. B. & Day, A. S. The role of S100A12 as a systemic marker of inflammation. Int. J. Inflam. 2012, 907078 (2012).
Yammani, R. R. et al. Interleukin-7 stimulates secretion of S100A4 by activating the JAK-STAT signaling pathway in human articular chondrocytes. Arthritis Rheum. 60, 792–800 (2009).
Donato, R. et al. Functions of S100 proteins. Curr. Mol. Med. 13, 24–57 (2013).
Yammani, R. R. et al. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 54, 2901–2911 (2006).
Van den Berg, W. B. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthritis Cartilage 19, 338–341 (2011).
Van Lent, P. L. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 58, 3776–3787 (2008).
Nacken, W. et al. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 60, 569–580 (2003).
Vogl, T. et al. Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes. Int. J. Mol. Sci. 13, 2893–2917 (2012).
Van Lent, P. L. et al. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 67, 1750–1758 (2008).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2008).
Schelbergen, R. F. P. et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like Receptor 4. Arthritis Rheum. 64, 1477–1487 (2012).
Cunningham, C. C. et al. Osteoarthritis-associated basic calcium phosphate crystals induce pro-inflammatory cytokines and damage-associated molecules via activation of Syk and PI3 kinase. Clin. Immunol. 144, 228–236 (2012).
Schelbergen, R. F. P. et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis. 75, 218–225 (2014).
Van Den Bosch, M. H. et al. The alarmins S100A8/A9 induce canonical Wnt signaling in murine knee joints; implications for osteoarthritis. Arthritis Rheumatol. 68, 152–163 (2016).
Schelbergen, R. F. P. et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann. Rheum. Dis. 74, 2254–2258 (2015).
Miller, R. et al. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheum. 67, 2933–2943 (2015).
Song, C. et al. Regulation of inflammatory response in human chondrocytes by lentiviral mediated RNA interference against S100A10. Inflamm. Res. 61, 1219–1227 (2012).
Swisher, J. F., Burton, N., Bacot, S. M., Vogel, S. N. & Feldman, G. M. Annexin A2 tetramer activates human and murine macrophages through TLR4. Blood 115, 549–558 (2010).
Cecil, D. L. & Terkeltaub, R. Transamidation by transglutaminase 2 transforms S100A11 calgranulin into a procatabolic cytokine for chondrocytes. J. Immunol. 180, 8378–8385 (2008).
Kosaki, A. et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J. Clin. Endocrinal. Metab. 89, 5423–5428 (2004).
Nakashima, M. et al. Role of S100A12 in the pathogenesis of osteoarthritis. Biochem. Biophys. Res. Commun. 422, 508–514 (2012).
Han, M. et al. Identification of osteoarthritis biomarkers by proteomic analysis of synovial fluid. J. Int. Med. Res. 40, 2243–2250 (2012).
Loeser, R. F. et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 52, 2376–2385 (2005).
Magna, M. & Pisetsky, D. S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 20, 138–146 (2014).
Li, Z. C. et al. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Invest. Med. 34, 298–303 (2011).
Amin, A. R. & Islam, A. B. Genomic analysis and differential expression of HMG and S100A family in human arthritis: upregulated expression of chemokines, IL-8 and nitric oxide by HMGB1. DNA Cell Biol. 33, 550–565 (2014).
Terada, C. et al. Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1) in human osteoarthritic cartilage. Acta Med. Okayama 65, 369–377 (2011).
Kyostio-Moore, S. et al. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp. Med. 61, 346–355 (2011).
Garcia-Arnandis, I. et al. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology (Oxford) 49, 854–861 (2010).
Liu-Bryan, R. & Terkeltaub, R. Chondrocyte innate immune MyD88-dependent signaling drives pro-catabolic effects of the endogenous TLR2/TLR4 ligands LMW-HA and HMGB1. Arthritis Rheum. 62, 2004–2012 (2010).
Taniguchi, N. et al. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol. Cell. Biol. 27, 5650–5663 (2007).
Heinola, T. et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin. Exp. Rheumatol. 28, 511–518 (2010).
García-Arnandis, I. et al. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes. Arthritis Res. Ther. 12, R165 (2010).
Wähämaa, H. et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res. Ther. 13, R136 (2011).
Van Eden, W., van der Zee, R. & Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 5, 318–330 (2005).
Kubo, T. et al. Stress-induced proteins in chondrocytes from patients with osteoarthritis. Arthritis Rheum. 28, 1140–1145 (1985).
Takahashi, K. et al. Localization of heat shock protein in osteoarthritic cartilage. Scand. J. Rheumatol. 26, 368–375 (1997).
Terauchi, R. et al. Hsp70 prevents nitric oxide-induced apoptosis in articular chondrocytes. Arthritis Rheum. 48, 1562–1568 (2003).
Siebelt, M. et al. Hsp90 inhibition protects against biomechanically induced osteoarthritis in rats. Arthritis Rheum. 65, 2102–2112 (2013).
Lambrecht, S. Heat-shock proteins in stromal joint tissues: innocent bystanders or disease-initiating proteins? Rheumatology (Oxford) 53, 223–232 (2014).
Lambrecht, S. et al. Differential expression of αB-Crystallin and evidence of its role as a mediator of matrix gene expression in osteoarthritis. Arthritis Rheum. 60, 179–188 (2009).
Lambrecht, S. et al. Proteome characterization of human articular chondrocytes leads to novel insights in the function of small heat-shock proteins in chondrocyte homeostasis. Osteoarthritis Cartilage 18, 440–446 (2010).
Kirsch, T. et al. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage 8, 294–302 (2000).
Pfander, D., Swoboda, B. & Kirsch, T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am. J. Pathol. 159, 1777–1783 (2001).
Ea, H. K. et al. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann. Rheum. Dis. 67, 1617–1625 (2008).
Graff, R. D. et al. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 43, 1571–1579 (2000).
Kumahashi, N. et al. Correlation of changes in pain intensity with synovial fluid adenosine triphosphate levels after treatment of patients with osteoarthritis of the knee with high-molecular-weight hyaluronic acid. Knee 18, 160–164 (2011).
Berenbaum, F. et al. Concomitant recruitment of ERK1/2 and p38MAPK signalling pathway is required for ativation of cytoplasmic phapholipase A2 via ATP in articular chondrocytes. J. Biol. Chem. 278, 13680–13687 (2003).
Ryan, L. M. et al. ATP-induced chondrocalcinosis. Arthritis Rheum. 35, 1520–1525 (1992).
Guévremont, M. et al. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann. Rheum. Dis. 63, 636–643 (2004).
Ohshima, S. et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 48, 2788–2795 (2003).
Janelle-Montcalm, A. et al. Extracellular localization of galectin-3 has a deleterious role in joint tissues. Arthritis Res. Ther. 9, R20 (2007).
Hirsiger, S. et al. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012, 315941 (2012).
Goldring, M. B. et al. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2, 459–465 (2000).
Pelletier, J. P. et al. Are cytokines involved in osteoarthritic pathophysiology? Semin. Arthritis Rheum. 20 (Suppl. 2), 12–25 (1991).
Towle, C. A. et al. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage 5, 293–300 (1997).
McNulty, A. L. et al. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J. Orthop. Res. 31, 1039–1045 (2013).
Blain, E. J. et al.The effect of thymosin β4 on articular cartilage chondrocyte matrix metalloproteinase expression. Biochem. Soc. Trans. 30, 879–882 (2002).
Homandberg, G. A. et al. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage 6, 231–244 (1998).
Pichika, R. & Homandberg, G. A. Fibronectin fragments elevate nitric oxide (NO) and inducible NO synthetase (iNOS) levels in bovine cartilage and iNOS inhibitors block fibronectin fragment mediated damage and promote repair. Inflamm. Res. 53, 405–412 (2004).
Homandberg, G. A. & Hui, F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage culturedwith fibronectin fragments. Arch. Biochem. Biophys. 334, 325–331 (1996).
Hwang, H. S. et al. Fibronectin ragment induced expression of matrix metallo-proteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res. Ther. 17, 320 (2015).
Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 16, 197–205 (2006).
Jyang, D. et al. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 23, 435–461 (2007).
Ohno, S. et al. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFκB and p38 MAP kinase in articular chondrocytes. J. Biol. Chem. 281, 17952–17960 (2006).
Barreto, G. et al. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res. Ther. 17, 379 (2015).
Fuerst, F. C. et al. Regulation of MMP3 by laminin alpha 4 in human osteoarthritic cartilage. Scand. J. Rheumatol. 40, 494–496 (2011).
Schminke, B. et al. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am. J. Pathol. 186, 410–418 (2016).
Nakoshi, Y. et al. Regulation of tenascin-C expression by tumor necrosis factor-alpha in cultured human osteoarthritis chondrocytes. J. Rheumatol. 35, 147–152 (2008).
Patel, L. et al. Tenascin-C induces inflammatory mediators and matrix degradation in osteoarthritic cartilage. BMC Musculoskelet. Disord. 12, 164 (2011).
Chockalingam, P. S. et al. Tenascin-C levels in synovial fluid are elevated after injury to the human and canine joint and correlate with markers of inflammation and matrix degradation. Osteoarthritis Cartilage 21, 339–345 (2013).
Hasegawa, M. et al. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. J. Rheumatol. 31, 2021–2026 (2004).
Okamura, N. et al. Deficiency of tenascin-C delays articular cartilage repair in mice. Osteoarthritis Cartilage 18, 839–848 (2010).
Lohmander, L. S. et al. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 36, 1214–1222 (1993).
Lees, S. et al. Bioactivity in an aggrecan 32-mer fragment is mediated via Toll-like receptor 2. Arthritis Rheum. 67, 1240–1249 (2015).
Manson, J., Thiemermann, C. & Brohi, K. Trauma alarmins as activators of damage-induced inflammation. Br. J. Surg. 99, 12–20 (2012).
Zhou, S. J., Sun, Z. X. & Liu, J. Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand. J. Clin. Lab. Invest. 73, 344–348 (2013).
Priam, S. et al. Identification of soluble 14-3-3ε as a novel subchondral bone mediator involved in cartilage degradation in osteoarthritis. Arthritis Rheum. 65, 1831–1842 (2013).
Maksymowych, W. P. & Marotta, A. 14-3-3η: a novel biomarker platform for rheumatoid arthritis. Clin. Exp. Rheumatol. 32, 35–39 (2014).
Théry, C. et al. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Prakken, B., Albani, S. & Martini, A. Juvenile idiopathic arthritis. Lancet 377, 2138–2149 (2011).
Lee, D. M. & Weinblatt, M. E. Rheumatoid arthritis. Lancet 358, 903–911 (2001).
Choy, E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51 (Suppl. 5), v3–v11 (2012).
Muller-Ladner, U., Pap, T., Gay, R. E., Neidhart, M. & Gay, S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 1, 102–110 (2005).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Frosch, M. et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 43, 628–637 (2000).
Kessel, C., Holzinger, D. & Foell, D. Phagocyte-derived S100 proteins in autoinflammation: putative role in pathogenesis and usefulness as biomarkers. Clin. Immunol. 147, 229–241 (2013).
Choi, I. Y. et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann. Rheum. Dis. 74, 499–505 (2015).
Hammer, H. B. et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 150–154 (2010).
Foell, D. et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 42, 1383–1389 (2003).
Youssef, P. et al. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J. Rheumatol. 26, 2523–2528 (1999).
Foell, D. et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 50, 1286–1295 (2004).
Frosch, M. et al. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 48, 2622–2626 (2003).
Kane, D. et al. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum. 48, 1676–1685 (2003).
Kruithof, E. et al. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res. Ther. 7, R569–R580 (2005).
Imamichi, T. et al. Expression and cloning of migration inhibitory factor-related protein (MRP)8 and MRP14 in arthritis-susceptible rats. Biochem. Biophys. Res. Commun. 194, 819–825 (1993).
van Lent, P. et al. The inhibitory receptor FcγRII reduces joint inflammation and destruction in experimental immune complex-mediated arthritides not only by inhibition of FcγRI/III but also by efficient clearance and endocytosis of immune complexes. Am. J. Pathol. 163, 1839–1848 (2003).
Nabbe, K. C. et al. Coordinate expression of activating Fc γ receptors I and III and inhibiting Fc γ receptor type II in the determination of joint inflammation and cartilage destruction during immune complex-mediated arthritis. Arthritis Rheum. 48, 255–265 (2003).
van Lent, P. L. et al. S100A8 causes a shift toward expression of activatory Fcγ receptors on macrophages via Toll-like receptor 4 and regulates Fcγ receptor expression in synovium during chronic experimental arthritis. Arthritis Rheum. 62, 3353–3364 (2010).
Grevers, L. C. et al. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 63, 1365–1375 (2011).
Vogl, T. et al. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat. Commun. 5, 4593 (2014).
Hofmann, M. A. et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 3, 123–135 (2002).
Odink, K. et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 330, 80–82 (1987).
Wittkowski, H. et al. Effects of intra-articular corticosteroids and anti-TNF therapy on neutrophil activation in rheumatoid arthritis. Ann. Rheum. Dis. 66, 1020–1025 (2007).
Foell, D. & Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 50, 3762–3771 (2004).
Holzinger, D. et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 71, 974–980 (2012).
Moncrieffe, H. et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 52, 1467–1476 (2013).
Hammer, H. B., Fagerhol, M. K., Wien, T. N. & Kvien, T. K. The soluble biomarker calprotectin (an S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab. Arthritis Res. Ther. 13, R178 (2011).
Hamada, T. et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 58, 2675–2685 (2008).
Kokkola, R. et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 46, 2598–2603 (2002).
Taniguchi, N. et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48, 971–981 (2003).
Pullerits, R. et al. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 48, 1693–1700 (2003).
Kokkola, R. et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 48, 2052–2058 (2003).
Ostberg, T. et al. Oxaliplatin retains HMGB1 intranuclearly and ameliorates collagen type II-induced arthritis. Arthritis Res. Ther. 10, R1 (2008).
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496 (2007).
Lotze, M. T. & Tracey, K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 (2005).
Palmblad, K. et al. Morphological characterization of intra-articular HMGB1 expression during the course of collagen-induced arthritis. Arthritis. Res. Ther. 9, R35 (2007).
Parkkinen, J. & Rauvala, H. Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. Enhancement of t-PA-catalyzed plasminogen activation by amphoterin. J. Biol. Chem. 266, 16730–16735 (1991).
Yamoah, K. et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol. Endocrinol. 22, 1141–1153 (2008).
Gondokaryono, S. P. et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J. Leukoc. Biol. 82, 657–665 (2007).
Schierbeck, H. et al. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J. Rheumatol. 40, 1604–1613 (2013).
Park, S. Y. et al. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur. J. Immunol. 45, 1216–1227 (2015).
Hreggvidsdottir, H. S. et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 86, 655–662 (2009).
Qin, Y. et al. HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death Dis. 5, e1077 (2014).
Srivastava, P. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2, 185–194 (2002).
van Eden, W. et al. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331, 171–173 (1988).
Kamphuis, S. et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet 366, 50–56 (2005).
de Kleer, I. M. et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J. Immunol. 172, 6435–6443 (2004).
van Roon, J. A. et al.Stimulation of suppressive T cell responses by human but not bacterial 60-kD heat-shock protein in synovial fluid of patients with rheumatoid arthritis. J. Clin. Invest. 100, 459–463 (1997).
Macht, L. M. et al. Relationship between disease severity and responses by blood mononuclear cells from patients with rheumatoid arthritis to human heat-shock protein 60. Immunology 99, 208–214 (2000).
Prakken, B. J. et al. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 46, 1937–1946 (2002).
Palmer, G. & Gabay, C. Interleukin-33 biology with potential insights into human diseases. Nat. Rev. Rheumatol. 7, 321–329 (2011).
Talabot-Ayer, D. et al. Immune-mediated experimental arthritis in IL-33 deficient mice. Cytokine 69, 68–74 (2014).
Collins, L. V. et al Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004).
Hajizadeh, S. et al. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res. Ther. 5, 234–240 (2003).
Harty, L. C. et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 71, 582–588 (2012).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Hazeldine, J. et al. N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways. Injury 46, 975–984 (2015).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Kakimoto, K. et al.Suppressive effect of a neutrophil elastase inhibitor on the development of collagen-induced arthritis. Cell. Immunol. 165, 26–32 (1995).
Witter, J. et al. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 30, 519–529 (1987).
Powell, J. D. & Horton, M. R. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 31, 207–218 (2005).
Cs-Szabo, G., Roughley, P. J., Plaas, A. H. & Glant, T. T. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 38, 660–668 (1995).
Di Virgilio, F. et al.Leukocyte P2 receptors: a novel target for anti-inflammatory and anti-tumor therapy. Curr. Drug Targets Cardiovasc. Haematol. Disord. 5, 85–99 (2005).
Hammer, H. B. et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation anddamage in rheumatoid arthritis. Ann. Rheum. Dis. 66, 1093–1097 (2007).
Wittkowski, H. et al.S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 58, 3924–3931 (2008).
O'Reilly, S. Pound the alarm: danger signals in rheumatic diseases. Clin. Sci. (Lond.) 128, 297–305 (2015).
Srikrishna, G. et al. Carboxylated glycans mediate colitis through activation of NF-κB. J. Immunol. 175, 5412–5422 (2005).
Hofmann, M. A. et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 (1999).
Prakken, B. J. et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 101, 4228–4233 (2004).
Koffeman, E. C. et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 60, 3207–3216 (2009).
Acknowledgements
The authors were supported by INSERM, the University of Paris 6 (Pierre and Marie Curie), Arthritis R&D (ROAD: Research on Osteoarthritic Diseases) and the Agence Nationale de la Recherche. D.H. acknowledges support from the Interdisciplinary Centre of Clinical Research, Münster, Germany (Clinical Research Award CRA0) and the German Ministry for Education and Science (BMBF 01GM08100, AID-Net).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, to substantial discussions of its content, to writing the article and to reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.H. declares that he has received speaker's fees from Novartis. M.N., F.B. and C.J. declare no competing interests.
Rights and permissions
About this article
Cite this article
Nefla, M., Holzinger, D., Berenbaum, F. et al. The danger from within: alarmins in arthritis. Nat Rev Rheumatol 12, 669–683 (2016). https://doi.org/10.1038/nrrheum.2016.162
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.162
This article is cited by
-
New insights into inflammatory osteoclast precursors as therapeutic targets for rheumatoid arthritis and periodontitis
Bone Research (2023)
-
Psoriatic arthritis: review of potential biomarkers predicting response to TNF inhibitors
Inflammopharmacology (2023)
-
Inflammatory macrophages exacerbate neutrophil-driven joint damage through ADP/P2Y1 signaling in rheumatoid arthritis
Science China Life Sciences (2022)
-
Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways
Arthritis Research & Therapy (2021)
-
HMGB1, anti-HMGB1 antibodies, and ratio of HMGB1/anti-HMGB1 antibodies as diagnosis indicator in fever of unknown origin
Scientific Reports (2021)