Key Points
-
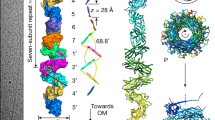
Chaperone–usher (CU) pili are assembled at the outer membrane by two proteins, a periplasmic chaperone, which provides the scaffold for the correct folding of pilins, and an outer-membrane protein called the usher, which forms a dimeric complex at the outer membrane to recruit and polymerize chaperone–subunit complexes and translocate the growing pilus to the outer surface. CU pili comprise linear, unbranched polymers of several hundreds to thousands of 12–20 kDa pilin subunits. This Review focuses on the rod-like fimbrial organelles, particularly uropathogenic Escherichia coli P and type 1 pili (the Pap and Fim systems, respectively).
-
Over the past decade, a plethora of structural information has been gathered about the various players that participate in P and type 1 pilus biogenesis, and these structural data, including the newly determined usher structures, are reviewed in detail.
-
Pilin subunits contain an incomplete, immunoglobulin-like fold that lacks the carboxy-terminal β-strand, which results in the presence of a large hydrophobic groove. When the chaperone–pilin complex is formed, a structural motif on the G1 strand of the chaperone is inserted into this groove. This donor strand complementation reaction is discussed in light of the most recent structural and biochemical data.
-
Pilin subunits also contain an amino-terminal extension (Nte) peptide that is disordered in the chaperone–subunit complex. During polymerization, the Nte on the incoming pilin subunit replaces the chaperone G1 strand, and this donor strand exchange reaction, including the 'zip-in, zip-out' mechanism, is also reviewed.
-
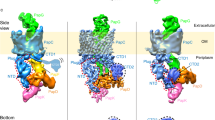
Recent structural data have indicated that only a single usher pore in the dimeric usher complex is used for pilus secretion. This model is discussed in detail.
-
Bacterial attachment to mucosal surfaces can be the result of the specific recognition of a pilus-associated adhesin by a host cell receptor. The molecular basis of receptor recognition of the FimH and PapG adhesins is described.
-
Finally, the complex role of CU pili in the pathogenesis of urinary tract infections is summarized, along with the prospects for using CU pili as a target for novel antibacterial therapies.
Abstract
The chaperone–usher (CU) pathway of pilus biogenesis is the most widespread of the five pathways that assemble adhesive pili at the surface of Gram-negative bacteria. Recent progress in the study of the structural biology of the CU pathway has unravelled the molecular basis of chaperone function and elucidated the mechanisms of fibre assembly at the outer membrane, leading to a comprehensive description of each step in the biogenesis pathway. Other studies have provided the molecular basis of host recognition by CU pili. The knowledge that has been gathered about both the assembly of and host recognition by CU pili has been harnessed to design promising antibiotic compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fronzes, R., Remaut, H. & Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 27, 2271–2280 (2008).
Sauer, F. G., Remaut, H., Hultgren, S. J. & Waksman, G. Fiber assembly by the chaperone-usher pathway. Biochim. Biophys. Acta 1694, 259–267 (2004).
Barnhart, M. M. et al. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl Acad. Sci. USA 97, 7709–7714 (2000).
Dodson, K. W., Jacob-Dubuisson, F., Striker, R. T. & Hultgren, S. J. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc. Natl Acad. Sci. USA 90, 3670–3674 (1993).
Thanassi, D. G. et al. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc. Natl Acad. Sci. USA 95, 3146–3151 (1998).
Nuccio, S. P. & Baumler, A. J. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 71, 551–575 (2007).
Kuehn, M. J., Heuser, J., Normark, S. & Hultgren, S. J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature 356, 252–255 (1992).
Baga, M., Norgren, M. & Normark, S. Biogenesis of E. coli Pap pili: PapH, a minor pilin subunit involved in cell anchoring and length modulation. Cell 49, 241–251 (1987).
Verger, D., Miller, E., Remaut, H., Waksman, G. & Hultgren, S. Molecular mechanism of P pilus termination in uropathogenic Escherichia coli. EMBO Rep. 7, 1228–1232 (2006).
Hahn, E. et al. Exploring the 3D molecular architecture of Escherichia coli type 1 pili. J. Mol. Biol. 323, 845–857 (2002).
Hultgren, S. J. et al. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc. Natl Acad. Sci. USA 86, 4357–4361 (1989).
Jones, C. H., Danese, P. N., Pinkner, J. S., Silhavy, T. J. & Hultgren, S. J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16, 6394–6406 (1997).
Vetsch, M. et al. Pilus chaperones represent a new type of protein-folding catalyst. Nature 431, 329–333 (2004).
Holmgren, A. & Branden, C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature 342, 248–251 (1989). This study elucidates the structure of a periplasmic chaperone.
Kuehn, M. J. et al. Structural basis of pilus subunit recognition by the PapD chaperone. Science 262, 1234–1241 (1993). This paper describes the first structure of a complex between a periplasmic chaperone and a peptide derived from a pilin subunit.
Slonim, L. N., Pinkner, J. S., Branden, C. I. & Hultgren, S. J. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 11, 4747–4756 (1992).
Choudhury, D. et al. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 285, 1061–1066 (1999).
Sauer, F. G. et al. Structural basis of chaperone function and pilus biogenesis. Science 285, 1058–1061 (1999). This article and reference 17 present the structures of chaperone–subunit complexes and formulate the concepts of donor strand complementation for chaperone function and donor strand exchange for subunit assembly. Reference 17 also provides insights into receptor recognition by the type 1 pilus, as it contains the structure of the FimH lectin domain.
Bann, J. G., Pinkner, J. S., Frieden, C. & Hultgren, S. J. Catalysis of protein folding by chaperones in pathogenic bacteria. Proc. Natl Acad. Sci. USA 101, 17389–17393 (2004).
Hung., D. L., Knight, S. D., Woods, R. M., Pinkner, J. S. & Hultgren, S. J. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 15, 3792–3805 (1996).
Zavialov, A. V. et al. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell 113, 587–596 (2003).
Remaut, H. et al. Donor-strand exchange in chaperone-assisted pilus assembly proceeds through a concerted β strand displacement mechanism. Mol. Cell 22, 831–842 (2006). This paper presents the first evidence that donor strand exchange occurs through a 'zip-in, zip-out' mechanism that is initiated at the P5 pocket.
Anderson, K. L. et al. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15, 647–657 (2004).
Sauer, F. G., Pinkner, J. S., Waksman, G. & Hultgren, S. J. Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell 111, 543–551 (2002). This paper and reference 21 reveal the structures of a ternary complex containing a subunit in donor strand complementation with its cognate chaperone and the same subunit in donor strand exchange with a second subunit. Together with reference 17, these studies validate the concept of donor strand exchange.
Vetsch, M. et al. Mechanism of fibre assembly through the chaperone-usher pathway. EMBO Rep. 7, 734–738 (2006).
Lindberg, F., Lund, B., Johansson, L. & Normark, S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature 328, 84–87 (1987).
Jacob-Dubuisson, F., Heuser, J., Dodson, K., Normark, S. & Hultgren, S. Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J. 12, 837–847 (1993).
Striker, R., Jacob-Dubuisson, F., Freiden, C. & Hultgren, S. J. Stable fiber-forming and nonfiber-forming chaperone-subunit complexes in pilus biogenesis. J. Biol. Chem. 269, 12233–12239 (1994).
Lee, Y. M., Dodson, K. W. & Hultgren, S. J. Adaptor function of PapF depends on donor strand exchange in P-pilus biogenesis of Escherichia coli. J. Bacteriol. 189, 5276–5283 (2007).
Rose, R. J. et al. Unraveling the molecular basis of subunit specificity in P pilus assembly by mass spectrometry. Proc. Natl Acad. Sci. USA 105, 12873–12878 (2008).
Verger, D. et al. Structural determinants of polymerization reactivity of the P pilus adaptor subunit PapF. Structure 16, 1724–1731 (2008).
Saulino, E. T., Thanassi, D. G., Pinkner, J. S. & Hultgren, S. J. Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J. 17, 2177–2185 (1998).
Nishiyama, M. et al. Structural basis of chaperone-subunit complex recognition by the type 1 pilus assembly platform FimD. EMBO J. 24, 2075–2086 (2005). This paper describes the first structure of the FimD N-terminal domain bound to a chaperone–subunit complex.
Nishiyama, M., Ishikawa, T., Rechsteiner, H. & Glockshuber, R. Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science 320, 376–379 (2008). This paper describes the first in vitro reconstitution of pilus biogenesis from purified components.
Remaut, H. et al. Fibre formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133, 640–652 (2008). This paper describes the structure of the translocation usher pore and proposes a general mechanism of usher function on the basis of the pore structure and a cryo-EM structure of the FimD usher bound to a secretion intermediate.
Li, H. et al. The outer membrane usher forms a twin-pore secretion complex. J. Mol. Biol. 344, 1397–1407 (2004).
So, S. S. & Thanassi, D. G. Analysis of the requirements for pilus biogenesis at the outer membrane usher and the function of the usher C-terminus. Mol. Microbiol. 60, 364–375 (2006).
Huang, Y., Smith, B. S., Chen, L. X., Baxter, R. H. & Deisenhofer, J. Insights into pilus assembly and secretion from the structure and functional characterization of usher PapC. Proc. Natl Acad. Sci. USA 106, 7403–7407 (2009).
Thanassi, D. G., Stathopoulos, C., Dodson, K., Geiger, D. & Hultgren, S. J. Bacterial outer membrane ushers contain distinct targeting and assembly domains for pilus biogenesis. J. Bacteriol. 184, 6260–6269 (2002).
Nishiyama, M., Vetsch, M., Puorger, C., Jelesarov, I. & Glockshuber, R. Identification and characterization of the chaperone-subunit complex-binding domain from the type 1 pilus assembly platform FimD. J. Mol. Biol. 330, 513–525 (2003).
Capitani, G., Eidam, O. & Grutter, M. G. Evidence for a novel domain of bacterial outer membrane ushers. Proteins 65, 816–823 (2006).
Ng, T. W., Akman, L., Osisami, M. & Thanassi, D. G. The usher N terminus is the initial targeting site for chaperone-subunit complexes and participates in subsequent pilus biogenesis events. J. Bacteriol. 186, 5321–5331 (2004).
Eidam, O., Dworkowski, F. S., Glockshuber, R., Grutter, M. G. & Capitani, G. Crystal structure of the ternary FimC-FimFt-FimDN complex indicates conserved pilus chaperone-subunit complex recognition by the usher FimD. FEBS Lett. 582, 651–655 (2008).
Munera, D., Palomino, C. & Fernandez, L. A. Specific residues in the N-terminal domain of FimH stimulate type 1 fimbriae assembly in Escherichia coli following the initial binding of the adhesin to FimD usher. Mol. Microbiol. 69, 911–925 (2008).
Munera, D., Hultgren, S. & Fernandez, L. A. Recognition of the N-terminal lectin domain of FimH adhesin by the usher FimD is required for type 1 pilus biogenesis. Mol. Microbiol. 64, 333–346 (2007).
Saulino, E. T., Bullitt, E. & Hultgren, S. J. Snapshots of usher-mediated protein secretion and ordered pilus assembly. Proc. Natl Acad. Sci. USA 97, 9240–9245 (2000).
Jacob-Dubuisson, F., Striker, R. & Hultgren, S. J. Chaperone-assisted self-assembly of pili independent of cellular energy. J. Biol. Chem. 269, 12447–12455 (1994).
Zavialov, A. V. et al. Resolving the energy paradox of chaperone/usher-mediated fibre assembly. Biochem. J. 389, 685–694 (2005).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Bahrani-Mougeot, F. K. et al. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45, 1079–1093 (2002).
Martinez, J. J., Mulvey, M. A., Schilling, J. D., Pinkner, J. S. & Hultgren, S. J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812 (2000).
Wu, X. R., Sun, T. T. & Medina, J. J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl Acad. Sci. USA 93, 9630–9635 (1996).
Hung., C. S. et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44, 903–915 (2002).
Merckel, M. C. et al. The structural basis of receptor-binding by Escherichia coli associated with diarrhea and septicemia. J. Mol. Biol. 331, 897–905 (2003).
Dodson, K. W. et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105, 733–743 (2001).
Roberts, J. A. et al. The Gal(α1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA 91, 11889–11893 (1994).
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 (2007).
Henderson, J. P. et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5, e1000305 (2009).
Bishop, B. L. et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nature Med. 13, 625–630 (2007).
Anderson, G. G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107 (2003).
Mulvey, M. A., Schilling, J. D. & Hultgren, S. J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69, 4572–4579 (2001).
Justice, S. S. et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl Acad. Sci. USA 101, 1333–1338 (2004).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103, 14170–14175 (2006).
Schilling, J. D., Mulvey, M. A., Vincent, C. D., Lorenz, R. G. & Hultgren, S. J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166, 1148–1155 (2001).
Linder, H., Engberg, I., Baltzer, I. M., Jann, K. & Svanborg-Eden, C. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect. Immun. 56, 1309–1313 (1988).
Hedges, S., Anderson, P., Lidin-Janson, G., de Man, P. & Svanborg, C. Interleukin-6 response to deliberate colonization of the human urinary tract with Gram-negative bacteria. Infect. Immun. 59, 421–427 (1991).
Mysorekar, I. U., Mulvey, M. A., Hultgren, S. J. & Gordon, J. I. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277, 7412–7419 (2002).
Mysorekar, I. U., Isaacson-Schmid, M., Walker, J. N., Mills, J. C. & Hultgren, S. J. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5, 463–475 (2009).
Langermann, S. et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276, 607–611 (1997).
Langermann, S. et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181, 774–778 (2000).
Kihlberg, J., Hultgren, S. J., Normark, S. & Magnusson, G. Probing the combining site of the PapG adhesin of uropathogenic Escherichia coli bacteria by synthetic analogues of galabiose. J. Am. Chem. Soc. 111, 6364–6368 (1989).
Ohlsson, J., Jass, J., Uhlin, B. E., Kihlberg, J. & Nilsson, U. J. Discovery of potent inhibitors of PapG adhesins from uropathogenic Escherichia coli through synthesis and evaluation of galabiose derivatives. Chembiochem 3, 772–779 (2002).
Pinkner, J. S. et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl Acad. Sci. USA 103, 17897–17902 (2006).
Wellens, A. et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE 3, e2040 (2008).
Bouckaert, J. et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 55, 441–455 (2005).
Hedenstrom, M. et al. NMR studies of interactions between periplasmic chaperones from uropathogenic E. coli and pilicides that interfere with chaperone function and pilus assembly. Org. Biomol. Chem. 3, 4193–4200 (2005).
Acknowledgements
This work was funded by Medical Research Council grant 85602 to G.W. and US National Institutes of Health grant 49950 to S.J.H.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Chaperone–usher pilus
-
A bacterial cell surface appendage that is assembled by the chaperone–usher pathway of pilus biogenesis.
- Curli
-
A type of fimbria that mediates binding to components of the extracellular matrix and is often implicated in biofilm formation.
- Type IV pilus
-
An elongated, flexible appendage that extends from the surface of Gram-negative bacterial cells and is used for adhesion and for cell motility (twitching motility).
- Type III secretion needle
-
A needle-like secretion apparatus in Gram-negative bacteria that forms pores in host membranes and allows the injection of virulence factors from the bacterial cytoplasm into the cytosol of host cells.
- Type IV secretion pilus
-
A pilus that is formed as part of the versatile secretion systems that are found in Gram-negative and Gram-positive bacteria. It can secrete a wide range of substrates, including protein–protein and protein–DNA complexes, and can directly target eukaryotic cells.
- Molecular dynamics
-
A form of computer simulation that calculates the time- dependent behaviour of atoms and molecules, providing information about the motion of the atoms and the resultant conformational changes in the molecules over time or during an interaction.
Rights and permissions
About this article
Cite this article
Waksman, G., Hultgren, S. Structural biology of the chaperone–usher pathway of pilus biogenesis. Nat Rev Microbiol 7, 765–774 (2009). https://doi.org/10.1038/nrmicro2220
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2220