Key Points
-
Marek's disease (MD) is a widespread, herpesvirus-induced neoplastic disease in the domestic chicken that is caused by Marek's disease virus (MDV). The main characteristic of MD is T-cell lymphomagenesis with dissemination of transformed cells preferentially to visceral organs and skeletal muscle, where solid tumours develop. Over the years, the virus has changed its tropism with a concomitant change in the clinical picture of MD, which now also results in oedema and widespread damage of the brain and central nervous system, ultimately leading to paralysis and death.
-
Three stages of MD infection can be distinguished. During the cytolytic phase, MDV enters the organism by inhalation from the environment, undergoes a first round of replication and disseminates to lymphoid organs; the virus then enters the latent stage of infection, predominantly in CD4+ T cells; finally, T-cell transformation, lymphomagenesis and spread of the lymphoid neoplasia ensues.
-
MDV encodes approximately 100 gene products, most of which have high similarity to those of related α-herpesviruses. Among the proteins unique to MDV are secreted factors, such as a viral homologue of chicken interleukin 8 (vIL-8) and a secreted protein with homology to pancreatic lipases (vLIP). These two secreted proteins, along with secreted glycoprotein C (gC), have important roles in the initial stages of infection, probably through cell signalling and the attraction of target cells.
-
MDV lymphomagenesis is a complex process, which appears to require robust lytic replication to ensure that the virus reaches activated T cells where latency and transformation commence. MDV-induced tumours, which are oligoclonal or monoclonal, mainly consist of latently infected T cells harbouring the integrated viral genome.
-
Transformation requires a basic leucine zipper protein, Meq, which is a member of the Jun/Fos family of oncoproteins. Meq possesses transforming properties in vitro, and a meq-negative MDV is unable to cause tumours.
-
MDV also encodes a functional and highly active viral telomerase RNA (vTR), which is expressed in the lytic, latent and tumour phase of infection and was shown to be required for efficient lymphomagenesis and to possess transforming properties.
-
MD is efficiently controlled by immunization using modified-live-virus vaccines. Despite the success of comprehensive vaccination, new virus variants are emerging which are able to break vaccine protection and exhibit increased virulence. Therefore, MD poses a constant threat to chicken populations worldwide.
-
Current research focuses on the elucidation of MD pathogenesis and the rational design and engineering of novel vaccines. For both these main areas of MD research, the generation of infectious virus clones and their mutagenesis in Escherichia coli are instrumental.
Abstract
Marek's disease virus (MDV) is an oncogenic herpesvirus that causes various clinical syndromes in its natural host, the chicken. MDV has long been of interest as a model organism, particularly with respect to the pathogenesis and immune control of virus-induced lymphoma in an easily accessible small-animal system. Recent advances in MDV genetics and the determination of the chicken genome sequence, aided by functional genomics, have begun to dramatically increase our understanding not only of lytic MDV replication, but also of the factors and mechanisms leading to latency and tumour formation. This new information is helping to elucidate cellular signalling pathways that have undergone convergent evolution and are perturbed by different viruses, and emphasizes the value of MDV as a comparative biomedical model. Furthermore, the door is now open for rational and efficient engineering of new vaccines against one of the most important and widespread infectious diseases in chickens.
Similar content being viewed by others
Main
The first description of Marek's disease (MD) dates from 1907, when József Marek, the pre-eminent clinician of the Budapest veterinary school after whom the disease was named in 1960, reported a generalized polyneuritis in four chickens1. Histological examination revealed that both the sciatic nerve and areas of the spinal cord were infiltrated with mononuclear cells, an observation that is still made today after infection with most Marek's disease virus (MDV) strains2,3. In the late 1920s, Pappenheimer and colleagues proposed that polyneuritis and visceral lymphoma were symptoms of the same disease4,5.
Marek's disease is similar to another neoplastic disease in chickens, retrovirus-induced lymphatic leukosis, and distinguishing between the two diseases was very difficult initially, although some clinical and pathological differences were apparent (reviewed in Ref. 6). A clear separation between retrovirus- and MDV-induced neoplasia became possible in the late 1960s, when the herpesvirus aetiology of MD was established. The identification and cell-culture isolation of MDV quickly led to the development of vaccines that achieved unparalleled success in preventing the disease and provided a landmark: the first effective immune prophylaxis against a cancer7,8,9,10,11,12. However, during the past 25 years, the changing nature of the disease and the increased virulence of MDV (Box 1) have led to concerns that new vaccines are now required (Box 2).
In this review, we summarize our current understanding of how MDV interferes with host defence mechanisms to initiate and complete lytic replication, establishes latent infection in target cells and, ultimately, transforms T cells. In addition, the advantages of the MDV–chicken system as a versatile small-animal model for studying herpesvirus pathogenesis and oncogenesis will be presented. These include the ability to investigate tumour formation in a natural virus–host setting in which lymphoma and solid tumours form with remarkable kinetics and reliability. We will emphasize how recent advances in MDV genomics now allow for targeted disruption of MDV genes, which have a role in evolutionary conserved pathways of transformation as well as tumour formation and dissemination.
Classification of MDV in the family Herpesviridae
Owing to its biological properties, particularly its ability to induce T-cell lymphoma and its slow growth in cell culture, MDV was long thought to be closely related to Epstein–Barr virus (EBV), a member of the Gammaherpesvirinae. Electron-microscopy studies of the MDV genome provided the first evidence that this double-stranded DNA virus possesses repeat structures that are characteristic of the Alphaherpesvirinae13, which was later confirmed by detailed restriction-enzyme mapping and sequencing, first of individual genes, and, later, entire genomes14,15,16. Therefore, MDV is genetically closely related to human herpesvirus 1 (herpes simplex virus type 1, HSV-1) and human herpesvirus 3 (varicella–zoster virus, VZV) (Fig. 1).
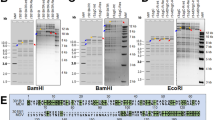
Comparison of the genomic organization of varicella–zoster virus (VZV) (a) with MDV (b). VZV is the closest relative of MDV affecting humans and the comparison shows significant homology, especially in unique regions of the genomes. The terminal and internal repeat long regions (TRL, IRL), the unique long region (UL), the internal and terminal repeat short regions (IRS, TRS) and the unique short region (US) are shown. The sequence similarity between the proteins encoded by the individual genes is colour coded. Note that only five VZV genes (depicted in white in the VZV sequence) are not present in MDV. The largest variation between the MDV and VZV genomes is the presence of large repeats bracketing the unique long region (TRL and IRL) in MDV. The TRL and IRL regions contain genes that encode proteins or RNA structures that are important for cellular tropism (for example, viral interleukin 8, vIL-8) as well as tumorigenicity (for example, Meq and vTR)15,16.
MDV belongs to the genus Mardivirus, into which three closely related but distinct species have been grouped: MDV (gallid herpesvirus type 2, GaHV-2), which is the type strain for the genus; GaHV-3 (previously referred to as MDV-2); and turkey herpesvirus 1 (HVT; meleagrid herpesvirus type 1, MeHV-1; previously MDV-3). The sequence similarity of virus proteins between the three viruses ranges from 50% to 80% (Refs 17–20). Only MDV causes clinical disease in chickens, the other two species are non-pathogenic. The genomes of MDV, GaHV-3 and HVT consist of a unique long (UL) and a unique short (US) segment bracketed by inverted repeats known as terminal and internal repeats long (TRL and IRL) and terminal and internal repeats short (TRS and IRS), respectively. The genes located in the UL and US segments are largely homologous to, and arranged collinearly with, those of HSV-1 and VZV, whereas genus- and virus-specific genes are located in the inverted repeat regions, predominantly TRL and IRL (Fig. 1) (Ref. 13).
MDV as a model for human disease
MDV has proven to be a valuable model organism for understanding some of the principles of human disease. It provides a well defined small-animal model of general tumorigenesis, and virus-induced lymphomagenesis in particular. Infection of birds with MDV strains of varying virulence causes tumours to form within a few weeks in chicken lines from many different genetic backgrounds21,22,23,24. Depending on the virulence of the virus strain and the genotype of the chicken, T-cell lymphomas and solid visceral tumours that contain transformed CD4+ T cells and immune effector cells such as macrophages, as well as MDV-specific B- and T cells, develop within two to six weeks of infection25,26. The reliable kinetics of disease induction and progression, together with the fact that lymphomagenesis can be studied in a natural virus–host system, make MDV unique and allow studies that are impossible to perform in other non-natural models of herpesvirus disease and oncogenesis, for example investigating the role of immune control and evasion in neoplasm formation.
Additionally, it has been recognized that the early stages of MDV infection of chickens closely mimic those of chickenpox in humans, which occurs following VZV infection of naive individuals. The similarities between the early pathogenesis of MD and chickenpox include the uptake of infectious virus by macrophages or dendritic cells (DCs) in the respiratory tract, the infection of activated CD4+ T cells and the development of cell-associated viraemia mainly in CD4+ T cells. Finally, free infectious virus, which is the means of virus transmission from one infected host to another, is produced only in terminally differentiated epithelial cells in feather (MDV) or hair (VZV) follicles26,27,28. It has also been shown that MDV and VZV require homologous viral gene products for efficient virus growth in cultured cells and in vivo (reviewed in Ref. 18).
Although the promise of MD as a model for biomedical research had long been recognized and exploited, MDV fell from favour, mainly because of the limited availability of the molecular tools that are necessary for efficient and reliable virus manipulation. In addition, there was a shortage of genetic information about the virus host, the chicken, as well as a lack of well defined reagents that would have allowed in-depth elucidation of the functional processes leading to MDV uptake, dissemination, latency and tumour formation. In the past five years, however, some of these major impediments have been removed. Several MDV strains have been cloned, either as bacterial artificial chromosomes (BACs) or as a set of overlapping cosmids, so straightforward genetic manipulation is now not only feasible but has already brought rapid gains in our molecular understanding of MDV. Specifically, the genomes of four MDV strains — three avirulent vaccine strains (584Ap80C, CVI988 and an avirulent form of strain Md11) and one very virulent (vv) strain (RB-1B; see Box 1) — are available as BACs, and the genome of vv strain Md5 has been established as a set of five overlapping cosmid clones29,30,31,32,33. With the cloned viral genomes and various Escherichia coli-based recombination systems at hand for manipulation, timely and efficient mutagenesis of MDV can be accomplished by both random and targeted approaches29,34,35,36,37. The ability to introduce markerless modifications, even in both copies of a given sequence in the repeat regions of the genome, has proven invaluable in the progress of MDV research (Fig. 2). Likewise, the completion of the chicken genome sequence and its ongoing annotation are major developments that will have a huge positive impact on MD research and the investigation of the molecular mechanisms of disease development and lymphomagenesis, especially if genetic polymorphisms of the host are considered38,39,40,41.
a | Infectious MDV clones. Three of the four MDV BACs that have been generated and described so far contain mini-F sequences (pHA1) instead of MDV094 (US2), which is dispensable for virus growth in vivo and tumorigenesis. The mini-F sequences in BAC20, pCVI988 and pRB-1B are flanked by loxP sites (red circles) that are amenable to Cre-mediated recombination, which allows vector sequences to be removed from the final virus construct29,30,32. b,c | The principle of Red (RecET) recombination, which exploits the DNA recombination machinery encoded by prophage Rac or phage λ. Red recombination requires double-strand breaks and short homology arms of only 30 to 50 base pairs. Linear DNA, usually a positive-selection marker amplified by PCR, is protected from degradation by the activity of Gam blocking RecBCD. The exonuclease RecE (Recα or Exo in phage λ) generates a 3′ overhang by processive degradation of the 5′ strand. The single-stranded-DNA-binding protein RecT (Recβ or Bet in phage λ) finally introduces the linear DNA fragment into a replication fork127,128. d | A two-step Red recombination that utilizes counter-selection of recombinants by utilizing homing endonucleases, such as I-SceI, allows markerless modifications such as point mutations and insertions of short or longer sequences. After a first Red recombination, which is selected for by screening for the presence of the positive selection marker, usually an antibiotic-resistance gene such as aphAI, which confers resistance to kanamycin, a second Red ('en passant') recombination then results in removal of the selection marker by utilizing a double-strand break generated by cleavage with I-SceI in close proximity to a sequence that had been duplicated129. IRL/S, internal repeats long/short region; TRL/S, terminal repeats long/short region; UL/S, unique long/short region.
The early cytolytic phase of MDV infection
Cellular tropism of MDV and early events after infection. MDV can persist for extended periods in the environment and is so ubiquitous that virtually every chicken worldwide faces MDV challenge from its first day of life. The first step of the MDV replication cycle in the definitive host is inhalation of the virus. The current model of MDV pathogenesis predicts that phagocytic cells in the respiratory tract — macrophages or DCs — become infected either directly or after an initial round of replication in epithelial cells26. Within 24 hours of uptake, virus is detectable in the spleen, thymus and the bursa of Fabricius42. Here, the virus meets its primary targets for the first phase of cytolytic replication: B cells and later activated CD4+ and, rarely, CD4−CD8− T cells or CD8+ T cells. The peak of virus replication in these cells is observed between three and seven days post infection (p.i.)43,44,45.
Infected CD4+ T cells not only serve as a target for transformation and as the reservoir for latent MDV genomes, they are also the means of virus spread within an infected animal, and allow transport to the skin. Infected T cells appear to be the 'Trojan horse' by which MDV enters the feather-follicle epithelium, where free infectious virus assembles and is shed to infect chickens that come into contact with the infected animal46,47,48.
A hallmark of lytic MDV infection is substantial and sustained downregulation of major histocompatibility complex (MHC) class I molecules on the surface of infected cells. The downregulation is encoded by an MDV early gene(s) and is maintained throughout the lytic cycle, resulting in virus-infected cells that are 'invisible' to the cytotoxic immune response carried out by CD8+ T cells49. MDV seems to be a promising model to evaluate the in vivo effects of MHC class I downregulation. It has recently been shown that the product of the UL49.5 gene, a protein that has previously been shown to be responsible for transporter associated with antigen processing (TAP) blockade and MHC class I downregulation in related varicelloviruses50, is involved in this mechanism of immune evasion (Fig. 3; Table 1). It is currently surmised that escape from MHC class I restricted cytotoxicity facilitates the establishment of, and reactivation from, latency and is important for virus dissemination. This escape allows MDV to reach epithelia in the liver, lung, kidneys, oesophagus, proventriculus (glandular stomach), adrenal gland and skin, organs where productive infection can be established26. It is unclear, however, whether virus spread to inner organs and skin during the second cytolytic phase is initiated by latently or lytically infected T cells. In this regard, the recent observation that VZV — at least in a SCIDhu model (a severe combined immunodeficient mouse reconstituted with human immune cells) — can reach the skin through infected memory CD4+ T cells within 24 hours of entering the circulation could be of great interest to MDV pathogenesis51. The relatively long period of time between infection of skin (that is, the presence of VZV-infected cells) and rash formation and virus spread can be explained by a subtle, well balanced response of the innate immune system induced by the presence of virus-infected cells. Although the expression of interferon-α (IFN-α) is downregulated in VZV-infected epidermal cells, the cells surrounding the originally infected cell develop a strong and sustained IFN-α response, which is constantly stimulated by the presence of VZV antigens and keeps the infection in check51. By analogy, the presence of MDV-infected cells in the skin early in the lytic stage of the disease is conceivable and would offer a new perspective on virus dissemination within infected animals and the spread of the virus to uninfected birds.
Shown are MDV gene products for which an essential function for growth in vitro or efficient growth in vivo has been shown. The membrane (glyco)proteins gB, gH/gL, gM/UL49.5p (MDV064), gE/gI, gK, UL31p/UL34p (MDV044, MDV047), as well as the tegument protein VP22 encoded by the U L49 homologous gene (MDV062), were shown to be essential for virus growth in vitro and — consequently — in vivo29,34,36,37,130,131. The major phosphoprotein pp38 (MDV073), which is phosphorylated by the serine/threonine protein kinase US3p encoded by MDV092, was shown to be essential for growth of virulent MDV in vitro (Table 1; Schumacher et al., unpublished data). The secreted factors viral interleukin-8 (vIL-8), viral lipase (vLIP) and glycoprotein C (gC) are required for full virulence of MDV in vivo53,55 (Table 1; Tischer et al., unpublished data). ER, endoplasmic reticulum.
Secreted MDV proteins and their role in lytic replication. During its long co-evolution with the chicken, MDV has developed strategies to efficiently recruit and infect target cells, some of which involve genes that were 'pirated' from the host and adapted for the benefit of the virus. In the TRL and IRL regions of the genome, MDV harbours a spliced gene (MDV003) that encodes a CXC chemokine of 18- to 20-kDa in size, referred to as viral interleukin-8 (vIL-8)15,16,52,53. MDV vIL-8, unlike its homologues in mice and humans, fails to attract chicken heterophils (corresponding to mammalian neutrophils), but is a potent chemoattractant for mononuclear cells53. Consistent with a role for MDV vIL-8 in attraction of target B and T cells as well as monocytes, deletion of the two vIL-8-encoding open reading frames resulted in mutant viruses that were severely impaired in their ability to cause efficient lytic replication, although two independently generated viruses devoid of vIL-8 were able to enter latency and retained the ability for oncogenic transformation, albeit at reduced efficiency53,54.
Another protein that has recently been shown to be important for efficient lytic replication of MDV in vivo is a large, 120-kDa N-glycosylated protein that is encoded by MDV010, a gene located at the extreme left terminus of the UL genome segment55. The predicted amino-acid sequence of the protein exhibits high similarity to a fowl-adenovirus protein of unknown function and, in a stretch of ∼200 amino acids, the α/β hydrolase fold of pancreatic lipases; the protein was therefore dubbed viral lipase (vLIP). The glycosylated protein is released into the supernatant of infected cells in apparently small quantities (Fig. 3), but is nonetheless required for efficient lytic virus replication in chickens55. The molecular mechanism of vLIP action is not known. Owing to a mutation of the critical acidic residue in the lipase catalytic triad, it is probable that the protein has lost enzymatic activity. It is tempting to speculate, however, that vLIP might still be able to bind to lipids, because the serine residue responsible for the nucleophilic attack on lipid substrates is conserved in the context of the appropriate amino-acid residues for formation of the oxyanion hole that is required for stable covalent-bond formation55. Current research is focused on identifying whether vLIP is able to specifically bind to cell-surface molecules on subpopulations of chicken peripheral blood mononuclear cells (PBMCs), either to initiate or inhibit signalling cascades that would result in attraction of target cells, increasing their susceptibility for infection, or generating a cytokine environment that is favourable for efficient MDV replication and dissemination.
MDV glycoprotein C (gC) — originally termed the A antigen56 — is secreted (Fig. 3) and is one of the major antigens to which the chicken immune system mounts a substantial serological response. Intriguingly, gC expression levels are greatly reduced in MDV attenuated by serial passage in culture, again a striking similarity to VZV, in which the same phenomenon is observed57. Why this type I transmembrane protein with a long hydrophobic C-terminal α-helix is secreted into the supernatant of infected cells (Fig. 3), and whether there is a direct correlation between reduced expression levels and attenuation, has been the focus of research in many laboratories for the past 30 years.
With regard to gC secretion, it was speculated that the short cytoplasmic tail would cause instability of the molecule and therefore secretion58, but recent data suggest that gC secretion is the result of the generation of a total of three splice variants, two of which encode glycoproteins devoid of a transmembrane domain (Table 1). The identification of alternative gC transcripts of different sizes is in agreement with the variability observed in the putative proteins encoded by UL44 (MDV057), which range in size from 44 kDa to 65 kDa58,59. Overexpression of gC in cultured cells led to a massive decrease in MDV replication, which was caused by a factor released into the supernatant of infected cells, presumably secreted forms of gC. By contrast, deletion of gC enhanced virus replication in cultured cells, but not in vivo59 (Tischer et al., unpublished data; Table 1).
The role of gC during replication of a virulent MDV in vivo was only recently addressed. A point mutation was introduced into the UL44 start codon in a BAC clone of the highly oncogenic vv strain RB-1B (pRB-1B)32. Growth of the resulting gC-negative virus in cultured cells was enhanced. Viral loads in peripheral blood after inoculation of birds were not significantly reduced compared with those detected for either the parental virus or a revertant virus in which the methionine start codon was restored (Table 1). The gC-negative mutant was severely impaired, however, in establishing latency and tumour formation, indicating that gC exerts its functions in MDV pathogenesis beyond lytic replication per se. These findings suggest a dual role for gC during MDV infection as both a membrane-bound protein facilitating virus spread and a secreted factor that might signal to target cells or interfere with the host's response to MDV infection. Point mutations in UL44 resulting in mutant viruses that can produce either membrane-bound gC or secreted gC exhibited different growth properties in cultured cells (Tischer et al., unpublished data; Table 1), and animal studies using these mutant viruses should help to dissect the effects of the various forms of this glycoprotein59.
The latent and tumour phase of MDV infection
Entry of MDV into latency. MDV enters the latent phase of infection from approximately 7 days p.i. Latency — defined as the presence and maintenance of viral genomes without production of infectious progeny virus — is mainly restricted to CD4+ T cells, although B cells, CD4−CD8− T cells and CD8+ T cells harbouring latent MDV have been isolated60,61,62,63,64,65. Relatively little is known about the sequence of events that leads to latent infection or determines the fate of a viral genome after infection of a susceptible cell. This 'black box' is not unique to MDV but is true for many herpesviruses, such as HSV-1, for which the viral proteins involved in establishment and reactivation from latency are known, but mechanistic details are sorely lacking66. In the case of MDV, we face another problem: discrimination of latently infected cells from transformed cells is nearly impossible, and the transitions and differences between the latent and transformed state are certainly not discrete — if there is coexistence of latency and transformation and the former does not by default result in the latter.
Estimates for the number of MDV genes that are transcribed in the latent (tumour) phase of infection are controversial and range from ∼10–30 (Refs 67–69). Part of the problem with determining the exact number of latent transcripts and proteins is that these investigations were carried out using lymphoblastoid cell lines (LCL) transformed with MDV, some of which are prone to spontaneous reactivation70. Unlike the precise number of truly latent transcripts, it is clear that most latency-associated transcripts originate in both the long and short repeat regions71.
Much of the work on MDV latency has concentrated on three regions: transcripts that are found in antisense orientation to MDV084 (ICP4), the so-called 1.8-kb family of transcripts, and transcripts originating from the meq (MDV004) gene region in the MDV EcoRI Q fragment of the genome (see below). In the MDV084 region, latency-associated transcripts (LATs) antisense to the MDV ICP4 homologue are detectable in LCL but are also at least partially present in lytically infected cells72,73,74. A virus mutant from strain RB-1B unable to express LATs was capable of robust lytic replication but failed to produce tumours after infection of susceptible animals. The results indicated that MDV latency is at least partially controlled by modulation of expression of one of its immediate-early genes; this is identical to the situation in many related viruses, for example HSV-1 and VZV73,75. Transcripts originating from a bidirectional promoter located in the IRL in close proximity to the IRL/UL junction are responsible for expression of one of the major phosphoproteins, pp38, expression of which is confined to the lytic cycle31,76, and — on the opposite strand — the 1.8-kb family of transcripts77,78. The role, temporal organization and potential cooperation of the 1.8-kb family of transcripts remain elusive, although two proteins encoded by these transcripts, a 7-kDa protein and a 14-kDa protein that is also detected as an immediate-early protein in lytic infection, have been shown to be involved in the induction and maintenance of MDV latency by RNA-interference experiments79,80,81,82,83.
MDV lymphomagenesis — the roles of Meq and vTR. The most important and obvious outcome of infection with MDV is transformation of infected cells followed by multifocal lymphoma formation4,5. The lymphocyte subpopulations transformed by the virus are identical to those in which latent infection is established, suggesting that latent infection is a prerequisite for oncogenic transformation and lymphomagenesis. Only a few members of the Herpesviridae are capable of inducing malignancies, and only EBV and human herpesvirus 6 (HHV-6) can integrate into the host genome70,84. Integration of MDV DNA into chicken chromosomes is common and seems to be random, as no recombination hot spots have been identified. It has been discovered, however, that the ends of integrated MDV genomes are elongated by the addition of telomeric repeats, suggesting a role for host telomeres, and possibly telomerase, in the process of integration (see below)70.
Efficient MDV-induced transformation of T cells seems to require a robust cytolytic infection because a sufficient number of latently infected cells must be generated to reliably induce lymphoma. At present, it is thought that only a small subset of latently infected T cells will proliferate and disseminate to generate neoplasms26. In fact, it was reported that tumours in a given bird are derived from few originally transformed cells, and might even be monoclonal in origin in some animals70,84. Despite the oligo- or monoclonality of tumours in individual birds, whether only a limited number of latently infected cells are transformed and will develop into lymphoma or whether selection for ultimately tumorigenic, malignant and widely disseminating lymphoma cells occurs after the transforming event remains to be elucidated.
Malignant transformation by MDV and the basic leucine zipper protein (bZIP) Meq, which has similarity to the oncoproteins Fos and Jun, are inseparable. Since the detection of meq85, researchers, mainly Kung and collaborators, have accumulated an impressive body of data dissecting the various functions of Meq, which include transactivation, DNA binding, chromatin remodelling and transcriptional regulation. Wild-type Meq is a 339-amino-acid protein that is expressed during both the lytic and the latent/tumour phase of infection86 (Fig. 4). Probably dependent on the status of infection, Meq can — through its leucine zipper — dimerize with itself, c-Jun, JunB and Fos, with interaction with c-Jun being favoured87. It has been proposed that the Meq–c-Jun interaction stabilizes the cellular protein, thereby allowing c-Jun to act more like the retroviral v-Jun oncoprotein, which has been shown to activate the cathepsin-like protein JTAP-1, JAC and the heparin-binding epidermal-growth-factor-like growth factor (HB-EGF), all proteins that are capable of independently transforming chicken cells. The upregulation of identical transformation-associated genes by the Meq–c-Jun heterodimer, together with the upregulation of anti-apoptotic factors such as Bcl-2 and c-Ski, the cellular homologue of retroviral v-Ski, strongly suggests convergent evolution of the transforming pathways of oncogenic avian retroviruses and herpesviruses88. In addition to homo- and heterodimerization with proto-oncoproteins, Meq can bind to several factors that are involved in cell-cycle control, including RB, p53 and cyclin-dependent kinase 2 (CDK2)86, which can also explain the role of Meq in oncogenic transformation of T cells (Fig. 4).
Two MDV gene products, Meq and the virus-encoded RNA subunit of telomerase, vTR, have been shown to be directly involved in MDV-induced lymphomagenesis. Meq is referred to as the 'MDV oncogene', and weak transformation of various cell types by Meq overexpression has been shown88,89. Meq forms homodimers, which bind to CRE/TRE elements and are trans-repressive, as are heterodimers between Meq and the C-terminal-binding protein (CtBP). In addition, Meq forms heterodimers with c-Myc, c-Fos, ATF and c-Jun, the latter being the strongest transactivator by binding to AP-1 sites, which results in upregulation of, for example, interleukin 2 (IL-2) and CD30. Meq was also shown to upregulate transcription of Ski, heparin-binding epidermal growth factor (HB-EGF), JAC and JTAP-1 (Refs 87,89,92, 132–134). The co-localization of Meq with cyclin-dependent kinase 2 (CDK2) in Cajal bodies as well as direct binding and sequestration of p53 and RB results in dysregulation of cell-cycle control89,133. The exact role of vTR in oncogenesis is unknown, but it was shown to be required for efficient tumour development and to impact on the aggressiveness of MDV-induced lymphomas (Table 1; Trapp et al., unpublished data). Proven interactions are indicated by solid lines, putative interactions by dashed lines.
One important function of the Meq–c-Jun heterodimer is transactivation by binding to promoters containing AP-1 or so-called MERE sites (Meq responsive elements that harbour CRE/TRE cores), resulting in upregulation of meq transcription and the transformation-associated genes described earlier. Likewise, Meq-induced upregulation of interleukin 2 and CD30 (Hodgkin's disease antigen, a member of the tumour-necrosis-factor receptor II family) expression through MERE sites was demonstrated89,90. The upregulation of CD30 in transformed cells is very interesting from a comparative viewpoint, because several human lymphomas, including Hodgkin's lymphoma, overexpress CD30. Furthermore, EBV latent membrane protein 1 (LMP-1) was shown to associate with TNF-receptor-associated factors TRAF-1, TRAF-2 and TRAF-3, which results in nuclear factor (NF)-κB activation, promotion of TH2 cytokine production, cell proliferation and upregulation of co-stimulatory molecules such as CD30. Although overexpression of CD30 in MDV-transformed cells is not caused by NF-κB activation, interference with the CD30 pathway seems to be a common mechanism shared by these two oncogenic herpesviruses89,90. Based on these observations, it has been speculated that CD30 overexpression is evolutionarily conserved and defines a particular subset of neoplasms. Moreover, immunization against CD30 might be an effective anti-cancer therapy91, and the MDV–chicken model provides an excellent experimental platform to further investigate such possible approaches to lymphoma control.
Does Meq have transforming capacity? And what is its exact role in lytic virus replication, latency, oncogenic transformation and virulence? The first question was addressed by attempts to transform or immortalize different cell types using Meq-expressing plasmids. Although Meq-induced transformation of chicken T cells has not yet been demonstrated, Meq clearly had transforming properties in continuous Rat-2 and chicken fibroblast DF-1 cell lines88,92. The second question was partially answered by recent studies using a meq-negative mutant derived from a vv strain, Md5; it was shown that the meq-negative mutant did not cause tumours in infected birds93. Given that an appreciable reduction in lytic virus replication was observed, and deletion of the entire meq gene might have an impact on other transcripts and proteins — such as a Meq–vIL-8 fusion protein94 — originating from this transcriptionally highly active region of the genome, it could not be concluded that the inability of the mutant virus to cause tumours was solely dependent on the absence of Meq.
Very recently, however, further confirmation of an essential role for Meq in transformation and oncogenesis was provided95. Meq possesses a Pro-Leu-Asp-Leu-Ser (PLDLS) motif that is known to bind the C-terminal-binding protein (CtBP), a cellular transcriptional co-repressor with important roles in the regulation of development and oncogenesis96. Strikingly, Meq shares this CtBP-binding motif with adenoviral oncoprotein E1A and EBV nuclear antigens EBNA-3A and EBNA-3C (Ref. 96). Previous reverse-genetics studies indicated that both EBNA3A and EBNA3C are crucial for efficient transformation of human B cells by EBV97,98, and it has been shown that the CtBP-binding domain of EBNA-3A has a crucial, albeit non-essential, role in sustaining proliferation of EBV-transformed LCLs99. Meq can interact with CtBP physically and functionally through its PLDLS motif, and this interaction is crucial for MDV-induced lymphomagenesis, as mutations in the CtBP interaction domain completely abolish oncogenicity of an RB-1B-based PLDLS-negative mutant virus95. These results highlight the convergent evolution of molecular mechanisms of herpesvirus-induced tumorigenesis and show that MD is an excellent experimental model to study the role of CtBP in oncogenic transformation.
With respect to the increased virulence of recently isolated MDV strains (Box 1), it is notable that Meq variants have been discovered. It has been known for some time that insertions of up to 59 amino acids, comprising a variable number of repeats of four prolines (PPPP) in the proline-rich central region of the protein, are found in strains of low virulence100,101. By contrast, more virulent strains exhibit modifications of the repeated PPPP motif in which the second proline is changed to alanine or glutamic acid100; the impact of these alterations on MDV virulence and its host range remains to be determined and is the focus of ongoing studies in several laboratories.
Despite the fact that Meq has a clear role in oncogenesis, its transforming properties — in comparison to retroviral oncoproteins such as v-Src — are weak. In this context, the question of the involvement of cellular and/or viral cofactors in virus-induced transformation seems logical. An interesting discovery was made in the IRL/TRL region of the MDV genome, namely the existence of a viral homologue of telomerase RNA (TR), termed vTR, which has extensive secondary structures that are similar to those of the so-called EBERs (Epstein-Barr encoded RNAs), small RNAs that are produced during latent EBV infection102. Together with a protein subunit with reverse-transcriptase activity (TERT), TR forms the functional core complex of the ribonucleoprotein enzyme telomerase. Telomerase activity is crucially involved in maintaining the physical integrity of eukaryotic chromosomes and has been implicated in cellular immortalization and tumorigenesis. MDV vTR has 88% sequence identity to chicken telomerase RNA (chTR) and was pirated from the chicken genome. Functional analyses have shown that vTR can reconstitute telomerase activity by interacting with chicken TERT (chTERT) more efficiently than chTR103. By contrast, a single-nucleotide mutation in the vTR sequence in the widely used vaccine strain CVI988-Rispens resulted in a substantial loss of functionality of vTR, strengthening the case for an association between vTR and the oncogenicity of MDV102,103.
vTR has an important role in MDV-induced T-cell lymphomagenesis, as shown by generating and analysing mutant viruses that lacked either one or both copies of the diploid vTR gene (Trapp et al., unpublished observations; Fig. 4; Table 1). vTR-negative mutants from the highly oncogenic MDV strain RB-1B were significantly impaired in their ability to induce lymphomas in MDV-susceptible birds, and tumour incidences were reduced by >60% when compared with parental virus or mutants lacking only one copy of vTR. Strikingly, solid neoplasms in individual birds infected with the vTR-negative viruses were also significantly smaller in size and less disseminated. Although tumour formation was clearly impaired in the absence of vTR, chickens still succumbed to chronic MD, as demonstrated by wasting of birds (Table 1). The detailed molecular mode of action of vTR in MDV pathogenesis remains to be revealed, but it clearly possesses transforming properties. Currently, it is speculated that vTR might have a role in genome integration by aiding in the generation of telomeric elongations at the ends of the viral genome as a prerequisite for integration, in enhancing the survival of (latently) infected cells by its anti-apoptotic properties, and/or in promoting tumour-cell dissemination and homing to various organs by upregulation of cell-surface adhesion molecules such as integrin-αv104.
Conclusions
Almost a century after the first description of Marek's disease, research on this syndrome, which is prevalent worldwide, is at a crossroads. The necessary tools are now available to allow in-depth characterization of the molecular events leading to primary infection, lytic replication, the establishment and maintenance of the latent state and, eventually, tumour formation and dissemination. MD research is therefore poised to have an important impact on fundamental cancer biology, particularly with regard to the pathogenesis of, and intervention in, aggressive virus-induced lymphoma. Special emphasis will be placed on elucidating the molecular details of T-cell transformation and the factors that govern tumour formation and dissemination in this natural virus–host model. Future research will certainly focus on the roles of Meq, vTR and possibly other virus-encoded small RNA structures such as microRNAs, which have been shown to be associated with latency and/or transformation in other (herpes)virus systems105,106,107.
It is probable that the biggest challenge that lies ahead of the field, however, is the justified concern that the vaccines currently in use will soon reach the end of their useful lives. It is widely accepted that classical methods of improving MD vaccines “approach the threshold of efficacy” as stated by Witter, one of the founding fathers of MD vaccinology108. The only way out of this quandary is the rational design of a new generation of modified-live-virus vaccines as well as the development of more efficacious vaccine formulations and regimens. The recently generated infectious clones from different MDV strains will be instrumental in reaching this goal.
References
Marek, J. Multiple Nervenentzündung (Polyneuritis) bei Hühnern. Dtsch. Tierärztl. Wochenschr. 15, 417–421 (1907). The first description of the disease.
Bacon, L. D., Witter, R. L. & Silva, R. F. Characterization and experimental reproduction of peripheral neuropathy in White Leghorn chickens. Avian Pathol. 30, 487–499 (2001).
Gimeno, I. M., Witter, R. L. & Reed, W. M. Four distinct neurologic syndromes in Marek's disease: effect of viral strain and pathotype. Avian Dis. 43, 721–737 (1999).
Pappenheimer, A. W., Dunn, L C. & Cone, V. Studies on fowl paralysis (Neurolymphomatosis gallinarum). I. Clinical features and pathology. J. Exp. Med. 46, 63–86 (1929).
Pappenheimer, A. W., Dunn, L. C. & Seidlin, S. M. Studies on fowl paralysis (Neurolymphomatosis gallinarum). II. Transmission experiments. J. Exp. Med. 49, 87–102 (1929). In two back-to-back papers, the early findings of Marek are extended and a link is established between polyneuritis and visceral lymphoma. In addition, the first description of the infectious nature of the disease.
Biggs, P. M. History of Marek's disease. In Marek's Disease (ed. Hirai, K.) 1–24 (Springer, Berlin, 2001).
Churchill, A. E., Payne, L. N. & Chubb, R. C. Immunization against Marek's disease using a live attenuated virus. Nature 221, 744–747 (1969). The use of attenuated MDV as a highly effective antitumour vaccine is described. This report is the first description of a highly efficient antitumour vaccine only two years after the identification and isolation of the agent.
Churchill, A. E. & Biggs, P. M. Herpes-type virus isolated in cell culture from tumors of chickens with Marek's disease. II. Studies in vivo. J. Natl Cancer Inst. 41, 951–956 (1968).
Churchill, A. E. Herpes-type virus isolated in cell culture from tumors of chickens with Marek's disease. I. Studies in cell culture. J. Natl Cancer Inst. 41, 939–950 (1968).
Churchill, A. E. & Biggs, P. M. Agent of Marek's disease in tissue culture. Nature 215, 528–530 (1967). This paper describes the isolation and unequivocal identification of the MD agent as a herpesvirus.
Epstein, M. A., Achong, B. G., Churchill, A. E. & Biggs, P. M. Structure and development of the herpes-types virus of Marek's disease. J. Natl Cancer Inst. 41, 805–820 (1968).
Okazaki, W., Purchase, H. G. & Burmester, B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 14, 413–429 (1970).
Cebrian, J., Kaschka-Dierich, C., Berthelot, N. & Sheldrick, P. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc. Natl Acad. Sci. USA 79, 555–558 (1982).
Fukuchi, K., Sudo, M., Lee, Y. S., Tanaka, A. & Nonoyama, M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J. Virol. 51, 102–109 (1984). The starting point for MDV genetics and further confirmation of the virus' classification in the α -herpesvirus subfamily.
Tulman, E. R. et al. The genome of a very virulent Marek's disease virus. J. Virol. 74, 7980–7988 (2000).
Lee, L. F. et al. The complete unique long sequence and the overall genomic organization of the GA strain of Marek's disease virus. Proc. Natl Acad. Sci. USA 97, 6091–6096 (2000).
Kingham, B. F. et al. The genome of herpesvirus of turkeys: comparative analysis with Marek's disease viruses. J. Gen. Virol. 82, 1123–1135 (2001).
Osterrieder, N. & Vautherot, J. F. The genome content of Marek's disease virus. In Marek's Disease (eds Davison, T. F. & Nair, V. K.) 17–31 (Elsevier, London, 2004).
Davison, A. Comments on the phylogenetics and evolution of herpesviruses and other large DNA viruses. Virus Res. 82, 127–132 (2002).
Davison, A. J. Evolution of the herpesviruses. Vet. Microbiol 86, 69–88 (2002).
Adldinger, H. K. & Calnek, B. W. Pathogenesis of Marek's disease: early distribution of virus and viral antigens in infected chickens. J. Natl Cancer Inst. 50, 1287–1298 (1973). Gives the first account of the shedding of MDV in the feather follicle epithelia. This finding was instrumental in understanding the epidemiology and spread of the disease.
Calnek, B. W. Influence of age at exposure on the pathogenesis of Marek's disease. J. Natl Cancer Inst. 51, 929–939 (1973).
Schat, K. A., Calnek, B. W., Fabricant, J. & Abplanalp, H. Influence of oncogenicity of Marek' disease virus on evaluation of genetic resistance. Poult. Sci. 60, 2559–2566 (1981).
De Boer, G. F., Groenendal, J. E., Boerrigter, H. M., Kok, G. L. & Pol, J. M. Protective efficacy of Marek's disease virus (MDV) CVI-988 CEF65 clone C against challenge infection with three very virulent MDV strains. Avian Dis. 30, 276–283 (1986).
Burgess, S. C. & Davison, T. F. Identification of the neoplastically transformed cells in Marek's disease herpesvirus-induced lymphomas: recognition by the monoclonal antibody AV37. J. Virol. 76, 7276–7292 (2002).
Calnek, B. W. Pathogenesis of Marek's disease. In Marek's Disease (ed. Hirai, K.) 25–55 (Springer, Berlin, 2001).
Arvin, A. M. Varicella-zoster virus. Clin. Microbiol. Rev. 9, 361–381 (1996).
Arvin, A. M. Varicella-zoster virus: overview and clinical manifestations. Semin. Dermatol. 15, 4–7 (1996).
Schumacher, D., Tischer, B. K., Fuchs, W. & Osterrieder, N. Reconstitution of Marek's disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 74, 11088–11098 (2000). This paper is the first description of an infectious MDV clone and the first characterization of an essential MDV gene. The establishment of various MDV strains as infectious clones in subsequent years has allowed a thorough elucidation of MDV replication and pathogenesis.
Petherbridge, L. et al. Replication-competent bacterial artificial chromosomes of Marek's disease virus: novel tools for generation of molecularly defined herpesvirus vaccines. J. Virol. 77, 8712–8718 (2003).
Reddy, S. M. et al. Rescue of a pathogenic Marek's disease virus with overlapping cosmid DNAs: use of a pp38 mutant to validate the technology for the study of gene function. Proc. Natl Acad. Sci. USA 99, 7054–7059 (2002).
Petherbridge, L. et al. Oncogenicity of virulent Marek's disease virus cloned as bacterial artificial chromosomes. J. Virol. 78, 13376–13380 (2004).
Niikura, M., Dodgson, J. & Cheng, H. Direct evidence of host genome acquisition by the α-herpesvirus Marek's disease virus. Arch. Virol. 9 Sep 2005 (doi: 10.1007/s00705-005-0633-7).
Dorange, F., Tischer, B. K., Vautherot, J. F. & Osterrieder, N. Characterization of Marek's disease virus serotype 1 (MDV-1) deletion mutants that lack U L 46 to U L 49 genes: MDV-1 U L 49, encoding VP22, is indispensable for virus growth. J. Virol. 76, 1959–1970 (2002).
Osterrieder, N. et al. Generation and exploitation of infectious bacterial artificial chromosome (BAC) clones of animal herpesviruses. Berl. Munch. Tierarztl. Wochenschr. 116, 373–380 (2003).
Schumacher, D., Tischer, B. K., Reddy, S. M. & Osterrieder, N. Glycoproteins E and I of Marek's disease virus serotype 1 are essential for virus growth in cultured cells. J. Virol. 75, 11307–11318 (2001).
Tischer, B. K., Schumacher, D., Messerle, M., Wagner, M. & Osterrieder, N. The products of the U L 10 (gM) and the U L 49.5 genes of Marek's disease virus serotype 1 are essential for virus growth in cultured cells. J. Gen. Virol. 83, 997–1003 (2002).
Hillier, L. W. et al. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432, 695–716 (2004).
Schmutz, J. & Grimwood, J. Genomes: fowl sequence. Nature 432, 679–680 (2004).
Wallis, J. W. et al. A physical map of the chicken genome. Nature 432, 761–764 (2004).
Wong, G. K. et al. A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature 432, 717–722 (2004).
Schat, K. A., Schinazi, R. F. & Calnek, B. W. Cell-specific antiviral activity of 1-(2-fluoro-2-deoxy-β-D-arabinofuranosyl)-5-iodocytosine (FIAC) against Marek's disease herpesvirus and turkey herpesvirus. Antiviral Res. 4, 259–270 (1984).
Shek, W. R., Calnek, B. W., Schat, K. A. & Chen, C. H. Characterization of Marek's disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells. J. Natl Cancer Inst. 70, 485–491 (1983).
Baigent, S. J. & Davison, T. F. Development and composition of lymphoid lesions in the spleens of Marek's disease virus-infected chickens: association with virus spread and the pathogenesis of Marek's disease. Avian Pathol. 28, 287–300 (1999).
Baigent, S. J., Ross, L. J. N. & Davison, T. F. Differential susceptibility to Marek's disease is associated with differences in number, but not phenotype or location, of pp38+ lymphocytes. J. Gen. Virol. 79, 2795–2802 (1998).
Carrozza, J. H., Fredrickson, T. N., Prince, R. P. & Luginbuhl, R. E. Role of desquamated epithelial cells in transmission of Marek's disease. Avian Dis. 17, 767–781 (1973).
Johnson, E. A., Burke, C. N., Fredrickson, T. N. & DiCapua, R. A. Morphogenesis of Marek's disease virus in feather follicle epithelium. J. Natl Cancer Inst. 55, 89–99 (1975).
Niikura, M. et al. MDV glycoprotein D is expressed in the feather follicle epithelium of infected chickens. Acta Virol. 43, 159–163 (1999).
Hunt, H. D. et al. Marek's disease virus down-regulates surface expression of MHC (B complex) class 1 (BF) glycoproteins during active but not latent infection of chicken cells. Virology 282, 198–205 (2001).
Koppers-Lalic, D. et al. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl Acad. Sci. USA 102, 5144–5149 (2005).
Ku, C. C. et al. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J. Exp. Med. 200, 917–925 (2004).
Peng, Q. H. & Shirazi, Y. Characterization of the protein product encoded by a splicing variant of the Marek's disease virus Eco-Q gene (Meq). Virology 226, 77–82 (1996).
Parcells, M. S. et al. Marek's disease virus (MDV) encodes an interleukin-8 homolog (vIL-8): characterization of the vIL-8 protein and a vIL-8 deletion mutant MDV. J. Virol. 75, 5159–5173 (2001). For the first time, the biological role of a secreted CXC chemokine of MDV is described. MDV vIL-8 is shown to be important for lytic virus replication in vivo by the attraction of MDV target cells but not heterophils.
Cui, X., Lee, L. F., Reed, W. M., Kung, H. J. & Reddy, S. M. Marek's disease virus-encoded vIL-8 gene is involved in early cytolytic infection but dispensable for establishment of latency. J. Virol. 78, 4753–4760 (2004).
Kamil, J. P. et al. vLIP, a viral lipase homologue, is a virulence factor of Marek's disease virus. J. Virol. 79, 6984–6996 (2005).
Isfort, R. J., Kung, H. J. & Velicer, L. F. Identification of the gene encoding Marek's disease herpesvirus A antigen. J. Virol. 61, 2614–2620 (1987).
Ross, L. J., Basarab, O., Walker, D. J. & Whitby, B. Serological relationship between a pathogenic strain of Marek's disease virus, its attenuated derivative and herpes virus of turkeys. J. Gen. Virol. 28, 37–47 (1975).
Isfort, R. J., Stringer, R. A., Kung, H. J. & Velicer, L. F. Synthesis, processing, and secretion of the Marek's disease herpesvirus A antigen glycoprotein. J. Virol. 57, 464–474 (1986).
Tischer, B. K. et al. High-level expression of Marek's disease virus glycoprotein C is detrimental to virus growth in vitro. J. Virol. 79, 5889–5899 (2005).
Calnek, B. W., Schat, K. A., Ross, L. J. & Chen, C. L. Further characterization of Marek's disease virus-infected lymphocytes. II. In vitro infection. Int. J. Cancer 33, 399–406 (1984).
Schat, K. A., Chen, C. L., Shek, W. R. & Calnek, B. W. Surface antigens on Marek's disease lymphoblastoid tumor cell lines. J. Natl Cancer Inst. 69, 715–720 (1982).
Schat, K. A., Chen, C. L., Calnek, B. W. & Char, D. Transformation of T-lymphocyte subsets by Marek's disease herpesvirus. J. Virol. 65, 1408–1413 (1991).
Lee, S. I., Ohashi, K., Morimura, T., Sugimoto, C. & Onuma, M. Re-isolation of Marek's disease virus from T cell subsets of vaccinated and non-vaccinated chickens. Arch. Virol. 144, 45–54 (1999).
Takagi, M., Ohashi, K., Morimura, T., Sugimoto, C. & Onuma, M. Analysis of tumor suppressor gene p53 in chicken lymphoblastoid tumor cell lines and field tumors. J. Vet Med. Sci. 60, 923–929 (1998).
Morimura, T., Ohashi, K., Sugimoto, C. & Onuma, M. Pathogenesis of Marek's disease (MD) and possible mechanisms of immunity induced by MD vaccine. J. Vet Med. Sci. 60, 1–8 (1998).
Efstathiou, S. & Preston, C. M. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 111, 108–119 (2005).
Silver, S., Tanaka, A. & Nonoyama, M. Transcription of the Marek's disease virus genome in a nonproductive chicken lymphoblastoid cell line. Virology 93, 127–133 (1979).
Tanaka, A., Silver, S. & Nonoyama, M. Biochemical evidence of the nonintegrated status of Marek's disease virus DNA in virus-transformed lymphoblastoid cells of chicken. Virology 88, 19–24 (1978).
Maray, T., Malkinson, M. & Becker, Y. RNA transcripts of Marek's disease virus (MDV) serotype-1 in infected and transformed cells. Virus Genes 2, 49–68 (1988).
Delecluse, H. J. & Hammerschmidt, W. Status of Marek's disease virus in established lymphoma cell lines: herpesvirus integration is common. J. Virol. 67, 82–92 (1993). The physical state of MDV in transformed cells was a matter of debate that was resolved in this paper. In a follow-up study, the same authors could also show that MDV tumours in one animal are derived from very few, sometimes a single, originally transformed cell.
Sugaya, K., Bradley, G., Nonoyama, M. & Tanaka, A. Latent transcripts of Marek's disease virus are clustered in the short and long repeat regions. J. Virol. 64, 5773–5782 (1990).
Cantello, J. L., Anderson, A. S. & Morgan, R. W. Identification of latency-associated transcripts that map antisense to the Icp4 homolog gene of Mareks-disease virus. J. Virol. 68, 6280–6290 (1994).
Cantello, J. L., Parcells, M. S., Anderson, A. S. & Morgan, R. W. Marek's disease virus latency-associated transcripts belong to a family of spliced RNAs that are antisense to the ICP4 homolog gene. J. Virol. 71, 1353–1361 (1997).
Li, D. S., Pastorek, J., Zelnik, V., Smith, G. D. & Ross, L. J. N. Identification of novel transcripts complementary to the Mareks-disease virus homolog of the Icp4 gene of herpes-simplex virus. J. Gen. Virol. 75, 1713–1722 (1994).
Morgan, R. W. et al. Marek's disease virus latency. In Marek's Disease (ed. Hirai, K.) 223–243 (Springer, Berlin, 2001).
Shigekane, B., Kawaguchi, Y., Shirakata, M., Sakaguchi, M. & Hirai, K. The bi-directional transcriptional promoters for the latency-relating transcripts of the pp38/pp24 mRNAs and the 1.8 kb-mRNA in the long inverted repeats of Marek's disease virus serotype 1 DNA are regulated by common promoter-specific enhancers. Arch. Virol. 144, 1893–1907 (1999).
Kopacek, J., Ross, L. J., Zelnik, V. & Pastorek, J. The 132 bp repeats are present in RNA transcripts from 1.8 kb gene family of Marek disease virus-transformed cells. Acta Virol 37, 191–195 (1993).
Gimeno, I. M. et al. The pp38 gene of Marek's disease virus (MDV) is necessary for cytolytic infection of B cells and maintenance of the transformed state but not for cytolytic infection of the feather follicle epithelium and horizontal spread of MDV. J. Virol. 79, 4545–4549 (2005).
Hayashi, M., Kawamura, T., Akaike, H., Arai, S. & Okui, T. Antisense oligonucleotide complementary to the BamHI-H gene family of Marek's disease virus induced growth arrest of MDCC-MSB1 cells in the S-phase. J. Vet Med. Sci. 61, 389–394 (1999).
Kawamura, M. et al. The inhibitory effects of oligonucleotides, complementary to Marek's disease virus mRNA transcribed from the BamHI-H region, on the proliferation of transformed lymphoblastoid cells, MDCC-MSB1. J. Gen. Virol. 72, 1105–1111 (1991).
Hong, Y. & Coussens, P. M. Identification of an immediate-early gene in the Mareks-disease virus long internal repeat region which encodes a unique 14-kilodalton polypeptide. J. Virol. 68, 3593–3603 (1994).
Hong, Y., Frame, M. & Coussens, P. M. A 14-kDa immediate-early phosphoprotein is specifically expressed in cells infected with oncogenic Mareks-disease virus-strains and their attenuated derivatives. Virology 206, 695–700 (1995).
Peng, F. Y., Specter, S., Tanaka, A. & Nonoyama, M. A 7-kDa protein encoded by the BamhI-H gene family of Mareks-disease virus is produced in lytically and latently infected-cells. Int. J. Oncol. 4, 799–802 (1994).
Delecluse, H. J., Schuller, S. & Hammerschmidt, W. Latent Marek's disease virus can be activated from its chromosomally integrated state in herpesvirus-transformed lymphoma cells. EMBO J. 12, 3277–3286 (1993).
Jones, D., Lee, L., Liu, J. L., Kung, H. J. & Tillotson, J. K. Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proc. Natl Acad. Sci. USA 89, 4042–4046 (1992). This paper reports on the identification of Meq as a putative oncoprotein that is regularly expressed in lymphoblastoid cell lines.
Liu, J. L. et al. Functional interactions between herpesvirus oncoprotein MEQ and cell cycle regulator CDK2. J. Virol. 73, 4208–4219 (1999).
Qian, Z., Brunovskis, P., Lee, L., Vogt, P. K. & Kung, H. J. Novel DNA binding specificities of a putative herpesvirus bZIP oncoprotein. J. Virol. 70, 7161–7170 (1996).
Levy, A. M. et al. Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek's disease virus-transformed T cells. J. Virol. 77, 12841–12851 (2003).
Burgess, S. C. et al. Marek's disease is a natural model for lymphomas overexpressing Hodgkin's disease antigen (CD30). Proc. Natl Acad. Sci. USA 101, 13879–13884 (2004). In this paper, a link is made between Meq and overexpression of CD30, an important marker for Hodgkin's disease, underscoring the fact that MD is an excellent model for virus-induced oncogenesis.
Cheson, B. D. What is new in lymphoma? CA Cancer J. Clin. 54, 260–272 (2004).
Levy, A. M. et al. Marek's disease virus Meq transforms chicken cells via the v-Jun transcriptional cascade: a converging transforming pathway for avian oncoviruses. Proc. Natl. Acad. Sci. USA 102, 14831–14836 (2005).
Liu, J. L., Ye, Y., Lee, L. F. & Kung, H. J. Transforming potential of the herpesvirus oncoprotein MEQ: Morphological transformation, serum-independent growth, and inhibition of apoptosis. J. Virol. 72, 388–395 (1998).
Lupiani, B. et al. Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl Acad. Sci. USA 101, 11815–11820 (2004). A comprehensive and detailed analysis of the replication of MDV in vivo demonstrated that lytic replication is slightly reduced in the absence of Meq, but that tumours are completely absent, providing final proof of Meq's role in tumour formation in vivo.
Anobile, J. M. et al. Nuclear localization and dynamic properties of the Marek's disease virus oncogene products Meq and Meq/vIL8. J. Virol. 80, 1160–1166 (2006).
Brown, A. C. et al. Interaction of MEQ and CtBP is critical for induction of lymphomas by Marek's disease virus. Proc. Natl Acad. Sci. USA 103, 1687–1692 (2006).
Hickabottom, M., Parker, G. A., Freemont, P., Crook, T. & Allday, M. J. Two nonconsensus sites in the Epstein–Barr virus oncoprotein EBNA3A cooperate to bind the co-repressor carboxyl-terminal-binding protein (CtBP). J. Biol. Chem. 277, 47197–47204 (2002).
Maruo, S., Johannsen, E., Illanes, D., Cooper, A. & Kieff, E. Epstein–Barr virus nuclear protein EBNA3A is critical for maintaining lymphoblastoid cell line growth. J. Virol. 77, 10437–10447 (2003).
Tomkinson, B., Robertson, E. & Kieff, E. Epstein–Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 67, 2014–2025 (1993).
Maruo, S. et al. Epstein–Barr virus nuclear protein 3A domains essential for growth of lymphoblasts: transcriptional regulation through RBP-Jκ/CBF1 is critical. J. Virol. 79, 10171–10179 (2005).
Shamblin, C. E., Greene, N., Arumugaswami, V., Dienglewicz, R. L. & Parcells, M. S. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Vet. Microbiol. 102, 147–167 (2004).
Lee, S. I., Takagi, M., Ohashi, K., Sugimoto, C. & Onuma, M. Difference in the meq gene between oncogenic and attenuated strains of Marek's disease virus serotype 1. J. Vet. Med. Sci. 62, 287–292 (2000).
Fragnet, L., Blasco, M. A., Klapper, W. & Rasschaert, D. The RNA subunit of telomerase is encoded by Marek's disease virus. J Virol 77, 5985–5996 (2003). A virally encoded subunit of telomerase was identified and shown to be highly biologically active in a heterologous (murine) system. The first indication of such an RNA structure in tumour formation is given.
Fragnet, L., Kut, E. & Rasschaert, D. Comparative functional study of the viral telomerase RNA based on natural mutations. J. Biol. Chem. 280, 23502–23515 (2005).
Cayuela, M. L., Flores, J. M. & Blasco, M. A. The telomerase RNA component Terc is required for the tumour-promoting effects of Tert overexpression. EMBO Rep. 6, 268–274 (2005).
Pfeffer, S. et al. Identification of microRNAs of the herpesvirus family. Nature Methods 2, 269–276 (2005).
Pfeffer, S. et al. Identification of virus-encoded microRNAs. Science 304, 734–736 (2004).
Sullivan, C. S., Grundhoff, A. T., Tevethia, S., Pipas, J. M. & Ganem, D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature 435, 682–686 (2005).
Witter, R. L. & Kreager, K. S. Serotype 1 viruses modified by backpassage or insertional mutagenesis: approaching the threshold of vaccine efficacy in Marek's disease. Avian Dis. 48, 768–782 (2004).
Witter, R. L. Increased virulence of Marek's disease virus field isolates. Avian Dis. 41, 149–163 (1997). A detailed account of the changes in virulence of MDV strains over the decades is provided in a well standardized model. The relationship between use of MDV vaccines on the one hand and the increase in virulence and the change in tropism and disease manifestation on the other hand is discussed.
Witter, R. L. Avian tumor viruses: persistent and evolving pathogens. Acta Vet. Hung. 45, 251–266 (1997).
Witter, R. L. The changing landscape of Marek's disease. Avian Pathol. 27, S46–S53 (1998).
Gimeno, I. M. et al. Marek's disease virus infection in the brain: virus replication, cellular infiltration, and major histocompatibility complex antigen expression. Vet. Pathol. 38, 491–503 (2001).
Witter, R. L., Gimeno, I. M., Reed, W. M. & Bacon, L. D. An acute form of transient paralysis induced by highly virulent strains of Marek's disease virus. Avian Dis. 43, 704–720 (1999).
Jarosinski, K. W., Yunis, R., O'Connell, P. H., Markowski-Grimsrud, C. J. & Schat, K. A. Influence of genetic resistance of the chicken and virulence of Marek's disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis. 46, 636–649 (2002).
Jarosinski, K. W., Njaa, B. L., O'Connell, P. H. & Schat, K. A. Pro-inflammatory responses in chicken spleen and brain tissues after infection with very virulent plus Marek's disease virus. Vir. Immunol. 18, 148–161 (2005).
Witter, R. L., Nazerian, K., Purchase, H. G. & Burgoyne, G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am. J. Vet. Res. 31, 525–538 (1970). This paper, together with the initial isolation of the MDV agent in cultured cells, was the starting point for MD vaccination and the overwhelming success of a worldwide control programme.
King, D., Page, D., Schat, K. A. & Calnek, B. W. Difference between influences of homologous and heterologous maternal antibodies on response to serotype-2 and serotype-3 Marek's disease vaccines. Avian Dis. 25, 74–81 (1981).
Rispens, B. H., van Vloten, H., Mastenbroek, N., Maas, J. L. & Schat, K. A. Control of Marek's disease in the Netherlands. II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek's disease virus. Avian Dis. 16, 126–138 (1972).
Rispens, B. H., van Vloten, H., Mastenbroek, N., Maas, H. J. & Schat, K. A. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Dis. 16, 108–125 (1972). The authors isolated the first non-pathogenic variant of an MDV, which is currently the most efficacious vaccine and used worldwide.
Levy, A. M., Heller, E. D., Leitner, G. & Davidson, I. Effect of native chicken interferon on MDV replication. Acta Virol. 43, 121–127 (1999).
Markowski-Grimsrud, C. J. & Schat, K. A. Cytotoxic T lymphocyte responses to Marek's disease herpesvirus-encoded glycoproteins. Vet. Immunol. Immunopathol. 90, 133–144 (2002).
Nazerian, K., Witter, R. L., Lee, L. F. & Yanagida, N. Protection and synergism by recombinant fowl pox vaccines expressing genes from Marek's disease virus. Avian Dis. 40, 368–376 (1996).
Tischer, B. K. et al. A DNA vaccine containing an infectious Marek's disease virus genome can confer protection against tumorigenic Marek's disease in chickens. J. Gen. Virol. 83, 2367–2376 (2002).
Lee, L. F. et al. Characterization of a very virulent Marek's disease virus mutant expressing the pp38 protein from the serotype 1 vaccine strain CVI988/Rispens. Virus Genes 31, 73–80 (2005).
Cui, X. et al. A Marek's disease virus vIL-8 deletion mutant has attenuated virulence and confers protection against challenge with a very virulent plus strain. Avian Dis. 49, 199–206 (2005).
Suter, M. et al. BAC-VAC, a novel generation of (DNA) vaccines: a bacterial artificial chromosome (BAC) containing a replication-competent, packaging-defective virus genome induces protective immunity against herpes simplex virus 1. Proc. Natl Acad. Sci. USA 96, 12697–12702 (1999).
Narayanan, K., Williamson, R., Zhang, Y., Stewart, A. F. & Ioannou, P. A. Efficient and precise engineering of a 200 kb β-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene Ther. 6, 442–447 (1999).
Zhang, Y., Buchholz, F., Muyrers, J. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nature Genet. 20, 123–128 (1998).
Tischer, B. K., von Einem, J., Kaufer, B., Dan, T. & Osterrieder, N. Two-step Red-mediated recombination for versatile, high-efficiency markerless DNA manipulation in Escherichia coli. BioTechniques 40, 191–196 (2006).
Osterrieder, N. Sequence and initial characterization of the UL10 (glycoprotein M) and UL11 homologous genes of serotype 1 Marek's disease virus. Arch. Virol. 144, 1853–1863 (1999).
Schumacher, D., Tischer, B. K., Trapp, S. & Osterrieder, N. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 79, 3987–3997 (2005).
Liu, J. L. & Kung, H. J. Marek's disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style. Virus Genes 21, 51–64 (2000).
Liu, J. L. et al. MEQ and v-IL8: cellular genes in disguise? Acta Virol. 43, 94–101 (1999).
Qian, Z., Brunovskis, P., Rauscher, F., Lee, L. & Kung, H. J. Transactivation activity of Meq, a Mareks-disease herpesvirus bzip protein persistently expressed in latently infected transformed T-cells. J. Virol. 69, 4037–4044 (1995).
Jarosinski, K. W., Osterrieder, N., Nair, V. K. & Schat, K. A. Attenuation of Marek's disease virus by deletion of open reading frame RLORF4 but not RLORF5a. J. Virol. 79, 11647–11659 (2005).
Acknowledgements
We would like to thank the MDV community for many stimulating discussions and the privilege of being their colleagues. The past eight years of MDV research have been a rewarding experience. Special thanks go to H. Hunt and S. Burgess for critically reading the manuscript. Research on Marek's disease in our laboratory is supported by funds provided by the College of Veterinary Medicine at Cornell University and grants awarded through the National Research Initiative of the United States Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Polyneuritis
-
General inflammation of nerves.
- Visceral lymphoma
-
Solid neoplasms in organs of the abdomen such as heart, liver, kidney or gonads that are characterized by an accumulation of transformed lymphocytes and immune cells.
- Neoplastic disease
-
A disease involving uncontrolled cell growth, a cancer.
- Lymphomagenesis
-
Process involving the transformation and expansion of lymphocytes, resulting in a cancer of the affected cells.
- CD4+ T cells
-
A subpopulation of T cells that express the CD4 receptor. These cells aid in immune responses and are therefore referred to as T helper cells.
- Macrophages
-
Cells of the mononuclear phagocyte lineage that are responsible for phagocytosis of foreign material.
- Dendritic cells
-
(DCs). 'Professional' antigen-presenting cells that are found in the T-cell areas of lymphoid tissues and as minor cellular components in most tissues. They have a branched or dendritic morphology and are the most potent stimulators of T-cell responses.
- Bacterial artifical chromosome
-
(BAC). A prokaryotic cloning vector derived from a single-copy or low-copy-number mini-F plasmid that can stably maintain a large DNA insert (average size 150–300 kb) and can be propagated in Escherichia coli.
- Cosmid
-
A plasmid cloning vector containing two cohesive (cos) ends from phage λ and one or more selectable markers that allow efficient cloning and amplification of large DNA fragments (40–50 kb) in Escherichia coli.
- Bursa of Fabricius
-
The primary lymphoid organ in which B-cell maturation occurs in the chicken.
- CD4−CD8− T cells
-
T cells that express neither CD4 nor CD8 on their surface. These cells represent approximately 1–5% of αβ T cells and have been associated with immunoregulatory and immunosuppressive functions.
- CD8+ T cells
-
A subpopulation of T cells that express the CD8 receptor. CD8+ cells recognize antigens that are presented on the surface of host cells by MHC class I molecules, leading to their destruction, and are therefore also known as cytotoxic T cells.
- Immediate-early genes
-
Those herpesvirus genes encoding important transactivators that initiate and maintain the cascade-like expression of herpesvirus genes that also comprises early (mostly enzymes for DNA replication) and late (structural) genes.
- Transactivators
-
Proteins, such as the immediate-early genes or Meq, that function by enhancing the expression of other viral or cellular genes.
Rights and permissions
About this article
Cite this article
Osterrieder, N., Kamil, J., Schumacher, D. et al. Marek's disease virus: from miasma to model. Nat Rev Microbiol 4, 283–294 (2006). https://doi.org/10.1038/nrmicro1382
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1382
This article is cited by
-
Purinergic signaling during Marek’s disease in chickens
Scientific Reports (2023)
-
Critical roles of non-coding RNAs in lifecycle and biology of Marek’s disease herpesvirus
Science China Life Sciences (2023)
-
The genome evolution of Marek’s disease viruses in chickens and turkeys in China
Virus Genes (2023)
-
A highly pathogenic Marek’s disease virus isolate from chickens immunized with a bivalent vaccine in China
Archives of Virology (2022)
-
UL28 and UL33 homologs of Marek’s disease virus terminase complex involved in the regulation of cleavage and packaging of viral DNA are indispensable for replication in cultured cells
Veterinary Research (2021)