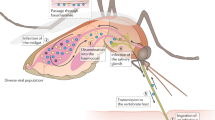
Key Points
-
Many recent viral pandemics have been attributed to the ability of some RNA viruses, for example HIV, dengue virus and possibly the severe acute respiratory syndrome (SARS) coronavirus, to change their host range to include humans. The authors discuss the mechanisms of host-range alteration used by a selection of viruses, including Venezuelan equine and Japanese encephalitis viruses (VEEV and JEV, respectively), dengue virus and West Nile virus (WNV).
-
Venezuelan equine encephalitis (VEE) was first recognized as a disease of horses, donkeys and mules in northern South America during the mid 1930s, but there has been renewed interest in this virus because of its potential as a biological weapon. Molecular analysis of epidemic strains — which exploit horses for amplification — and comparison with strains that do not cause epidemic disease, have shown that a few amino-acid mutations can affect host-range alteration. Changes on the surface of the VEE virion seem to be important for these host range changes.
-
JEV causes epidemics of encephalitis in India, Korea, China, South-East Asia and Indonesia. The disease affects children, and is associated with a mortality rate of greater than 20%. However, unlike VEEV, there is no evidence that JEV undergoes mutation and selection to replicate in different hosts. Pigs amplify transmission in peridomestic settings, and migratory birds have a role in dispersion of JEV. Although different genotypes have been isolated, their relevance to pathology and host range is unclear.
-
WNV is now endemic in the United States after first emerging in New York in 1999. WNV has a very broad host range. Forty-nine species of mosquitoes and ticks, and 225 species of birds are susceptible to infection. Other hosts include horses, cattle, llamas, alligators, cats, dogs, wolves and sheep. Transmission of WNV among these species has not been reported. Although humans are probably dead-end hosts, infection with WNV can cause severe disease.
-
Dengue viruses are very important human arboviral pathogens and use humans as reservoir hosts. Aedes aegypti and Aedes albopictus mosquitoes are the most common vectors in urban settings. It is thought that the human epidemic form of dengue virus evolved in the last 2000 years, and genetic analysis indicates that mutations have resulted in adaptation to the urban mosquito host. However, links between mutations and human pathogenicity have not been established.
-
Finally, the authors discuss how host-range changes can be studied experimentally. Cell-culture model systems can be used to find mutations that correlate with virus fitness and adaptation in different host strains. Viruses that replicate in useful laboratory animal models can also be studied in whole animal hosts.
Abstract
Many pandemics have been attributed to the ability of some RNA viruses to change their host range to include humans. Here, we review the mechanisms of disease emergence that are related to the host-range specificity of selected mosquito-borne alphaviruses and flaviviruses. We discuss viruses of medical importance, including Venezuelan equine and Japanese encephalitis viruses, dengue viruses and West Nile viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gao, F. et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436—441 (1999).
Korber, B. et al. Timing the ancestor of the HIV-1 pandemic strains. Science 288, 1789—1796 (2000).
Wang, E. et al. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J. Virol. 74, 3227—3234 (2000). This paper demonstrates the convergent evolution of endemic DENV virus strains from three of the four serotpes of sylvatic progenitors, and places a time frame on urban dengue emergence that is congruent with historical and epidemiological predictions.
Gubler, D. J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 33, 330—342 (2002).
Marra, M. A. et al. The genome sequence of the SARS-associated coronavirus. Science 300, 1399—1404 (2003).
Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276—278 (2003).
Rota, P. A. et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300, 1394—1399 (2003).
Rosenbloom, M., Leikin, J. B., Vogel, S. N. & Chaudry, Z. A. Biological and chemical agents: a brief synopsis. Am. J. Ther. 9, 5—14 (2002).
Kubes, V. & Rios, F. A. The causative agent of infectious equine encephalomyelitis in Venezuela. Science 90, 20—21 (1939).
Beck, C. E. & Wyckoff, R. W. G. Venezuelan equine encephalomyelitis. Science 88, 530 (1938).
Weaver, S. C., Ferro, C., Barrera, R., Boshell, J. & Navarro, J. Venezuelan equine encephalitis. Annu. Rev. Entomol. 49, 141—174 (2004).
Walton, T. E. & Grayson, M. A. in The Arboviruses: Epidemiology and Ecology Vol. IV (ed. Monath, T. P.) 203—231 (CRC, Boca Raton, Florida, 1988).
Walton, T. E., Alvarez, O., Buckwalter, R. M. & Johnson, K. M. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J. Infect. Dis. 128, 271—282 (1973).
Wang, E. et al. Virulence and viremia characteristics of 1992 epizootic subtype IC Venezuelan equine encephalitis viruses and closely related enzootic subtype ID strains. Am. J. Trop. Med. Hyg. 65, 64—69 (2001).
Rico-Hesse, R., Weaver, S. C., de Siger, J., Medina, G. & Salas, R. A. Emergence of a new epidemic/epizootic Venezuelan equine encephalitis virus in South America. Proc. Natl Acad. Sci. USA 92, 5278—5281 (1995).
Weaver, S. C. et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet 348, 436—440 (1996).
Oberste, M. S. et al. Association of Venezuelan equine encephalitis virus subtype IE with two equine epizootics in Mexico. Am. J. Trop. Med. Hyg. 59, 100—107 (1998).
Zarate, M. L., Scherer, W. F. & Dickerman, R. W. El virus de la encephalitis equina de Venezuela como determinante de infecciones en humanos, descripción de un caso fatal ocurrido en Jaltipán Veracruz en 1965. Rev. Invest. Salud Publica 30, 296—302 (1965).
Johnson, K. M. & Martin, D. H. Venezuelan equine encephalitis. Adv. Vet. Sci. Comp. Med. 18, 79—116 (1974).
Young, N. A. & Johnson, K. M. Antigenic variants of Venezuelan equine encephalitis virus: their geographic distribution and epidemiologic significance. Am. J. Epidemiol. 89, 286—307 (1969). This landmark paper established the antigenic relationships of enzootic and epizootic strains of VEEVs and provided the framework for future genetic studies that identified the origins of outbreaks.
Rico-Hesse, R., Roehrig, J. T., Trent, D. W. & Dickerman, R. W. Genetic variation of Venezuelan equine encephalitis virus strains of the ID variety in Colombia. Am. J. Trop. Med. Hyg. 38, 195—204 (1988).
Kinney, R. M., Tsuchiya, K. R., Sneider, J. M. & Trent, D. W. Genetic evidence that epizootic Venezuelan equine encephalitis (VEE) viruses may have evolved from enzootic VEE subtype I-D virus. Virology 191, 569—580 (1992).
Weaver, S. C., Bellew, L. A. & Rico-Hesse, R. Phylogenetic analysis of alphaviruses in the Venezuelan equine encephalitis complex and identification of the source of epizootic viruses. Virology 191, 282—290 (1992). This paper provides the first phylogenetic evidence that epizootic strains of VEEV have evolved repeatedly from enzootic subtype ID progenitors, and correctly predicts the occurrence of additional outbreaks after a 19-year absence.
Brault, A. C. et al. Potential sources of the 1995 Venezuelan equine encephalitis subtype IC epidemic. J. Virol. 75, 5823—5832 (2001).
Wang, E. et al. Genetic and phenotypic changes accompanying the emergence of epizootic subtype IC Venezuelan equine encephalitis viruses from an enzootic subtype ID progenitor. J. Virol. 73, 4266—4271 (1999).
Johnson, B. J., Brubaker, J. R., Roehrig, J. T. & Trent, D. W. Variants of Venezuelan equine encephalitis virus that resist neutralization define a domain of the E2 glycoprotein. Virology 177, 676—683 (1990).
Brault, A. C., Powers, A. M., Holmes, E. C., Woelk, C. H. & Weaver, S. C. Positively charged amino acid substitutions in the E2 envelope glycoprotein are associated with the emergence of Venezuelan equine encephalitis virus. J. Virol. 76, 1718—1730 (2002).
Holland, J. & Domingo, E. Origin and evolution of viruses. Virus Genes 16, 13—21 (1998).
Oberste, M. S., Schmura, S. M., Weaver, S. C. & Smith, J. F. Geographic distribution of Venezuelan equine encephalitis virus subtype IE genotypes in Central America and Mexico. Am. J. Trop. Med. Hyg. 60, 630—634 (1999).
Gonzalez-Salazar, D., Estrada-Franco, J. G., Carrara, A. S., Aronson, J. F. & Weaver, S. C. Equine amplification and virulence of subtype IE Venezuelan equine encephalitis viruses isolated during the 1993 and 1996 Mexican epizootics. Emerg. Infect. Dis. 9, 161—168 (2003).
Brault, A. C. et al. Venezuelan equine encephalitis emergence: Enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc. Natl Acad. Sci. USA 101, 11344—11349 (2004).
Ferro, C. et al. Natural enzootic vectors of Venezuelan equine encephalitis virus, Magdalena Valley, Colombia. Emerg. Infect. Dis. 9, 49—54 (2003).
Linthicum, K. J. et al. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285, 397—400 (1999).
Kramer, L. D. & Scherer, W. F. Vector competence of mosquitoes as a marker to distinguish Central American and Mexican epizootic from enzootic strains of Venezuelan encephalitis virus. Am. J. Trop. Med. Hyg. 25, 336—346 (1976).
Brault, A. C., Powers, A. M. & Weaver, S. C. Vector infection determinants of Venezuelan equine encephalitis virus reside within the E2 envelope glycoprotein. J. Virol. 76, 6387—6392 (2002).
Bowen, G. S. & Calisher, C. H. Virological and serological studies of Venezuelan equine encephalomyelitis in humans. J. Clin. Microbiol. 4, 22—27 (1976).
Weaver, S. C. in The Encyclopedia of Arthropod-transmitted Infections (ed. Service, M. W.) 151—159 (CAB International, Wallingford, UK, 2001).
Reisen, W. K. in The Encyclopedia of Arthropod-transmitted Infections (ed. Service, M. W.) 558—563 (CAB International, Wallingford, UK, 2001).
Hahn, C. S., Lustig, S., Strauss, E. G. & Strauss, J. H. Western equine encephalitis virus is a recombinant virus. Proc. Natl Acad. Sci. USA 85, 5997—6001 (1988).
Hiroyama, T. Epidemiology of Japanese encephalitis (in Japanese). Saishin-Igaku 17, 1272—1280 (1962).
Mitamura, T., Kitaoka, M., Mori, K. & Okuba, K. Isolation of Japanese epidemic encephalitis from mosquitoes caught in nature. Tokyo Iji Shinshi 62, 820—831 (1938).
Scherer, W. F. Ecological studies of Japanese encephalitis in Japan, parts I—IX. Am. J. Trop. Med. Hyg. 8, 644—722 (1959). This paper is one of a series of ten papers that describes the ecology, epidemiology and transmission cycles of JEV.
Tan, R., Nalim, S., Suwasono, H. & Jennings, G. B. Japanese encephalitis virus isolated from seven species of mosquitoes collected at Semarang Regency, Central Java. Bul. Penelit. Kesehatan 21, 1—5 (1993).
Chen, W. R., Tesh, R. B. & Rico-Hesse, R. Genetic variation of Japanese encephalitis virus in nature. J. Gen. Virol. 71, 2915—2922 (1990).
Chen, W. R., Rico-Hesse, R. & Tesh, R. B. A new genotype of Japanese encephalitis virus from Indonesia. Am. J. Trop. Med. Hyg. 47, 61—69 (1992).
Kobayashi, Y., Hasegawa, H. & Yamauchi, T. Studies on the antigenic structure of Japanese encephalitis virus using monoclonal antibodies. Microbiol. Immunol. 29, 1069—1082 (1985).
Sumiyoshi, H. et al. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology 161, 497—510 (1987).
Gould, E. A., de Lamballerie, X., Zanotto, P. M. & Holmes, E. C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv. Virus Res. 59, 277—314 (2003).
Solomon, T. et al. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 77, 3091—3098 (2003). This paper proposes that JEV originated in the Indonesia and Malaysia regions and has since spread to other parts of Asia and Australasia.
Hancock, J. & Kushlan, J. The Herons Handbook (Harper and Row, New York, 1984).
Hasegawa, H., Yoshida, M., Fujita, S. & Kobayashi, Y. Comparison of structural proteins among antigenically different Japanese encephalitis virus strains. Vaccine 12, 841—844 (1994).
Scherer, W. F., Buescher, E. L. & McClure, H. E. Ecologic studies of Japanese encephalitis virus in Japan. V. Avian factors. Am. J. Trop. Med. Hyg. 8, 689—697 (1959).
Work, T. H., Hurlbut, H. S. & Taylor, R. M. Indigenous wild birds of the Nile Delta as potential West Nile virus circulating reservoirs. Am. J. Trop. Med. Hyg. 4, 872—888 (1955).
Lanciotti, R. S. et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286, 2333—2337 (1999). This paper describes the emergence of WNV in the United States.
O'Leary, D. R. et al. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 4, 61—70 (2004).
Davis, C. T. et al. Genetic variation among geographically distinct West Nile virus isolates collected in the United States during 2002. Emerg. Infect. Dis. 9, 1423—1429 (2003).
Estrada-Franco, J. G. et al. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg. Infect. Dis. 9, 1604—1607 (2003).
Finney, S. Hopelessly lost, the homing pigeon whose cross-channel hop took him to New York. Daily Mail (Lond.) (23 June 2003).
Marra, P. P., Griffing, S. M. & McLean, R. G. West Nile virus and wildlife health. Emerg. Infect. Dis. 9, 898—899 (2003).
McLean, R. G. West Nile Virus: emerging threat to public health and animal health. J. Vet. Med. Educ. 30, 143—144 (2003).
Malkinson, M. & Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 267, 309—322 (2002).
Murgue, B., Zeller, H. & Deubel, V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr. Top. Microbiol. Immunol. 267, 195—221 (2002).
Intrauterine West Nile virus infection — New York, 2002. MMWR Morb. Mortal Wkly. Rep. 51, 1135—1136 (2002).
Detection, of West Nile virus in blood donations — United States, 2003. MMWR Morb. Mortal Wkly Rep. 52, 769—772 (2003).
Petersen, L. R., Roehrig, J. T. & Hughes, J. M. West Nile virus encephalitis. N. Engl. J. Med. 347, 1225—1226 (2002).
Pealer, L. N. et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 349, 1236—1245 (2003).
Iwamoto, M. et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 348, 2196—203 (2003).
Petersen, L. R., Marfin, A. A. & Gubler, D. J. West Nile virus. JAMA 290, 524—528 (2003).
Harrington, T. et al. West Nile virus infection transmitted by blood transfusion. Transfusion 43, 1018—1022 (2003).
Gause, G. F. The Struggle for Existence (Dover Publications, New York, 1971).
WHO. Dengue and dengue haemorrhagic fever. [online], <http://www.who.int/mediacentre/factsheets/fs117> (April, 2002).
Diallo, M. et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999—2000: entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 9, 362—367 (2003).
Rudnick, A. Studies of the ecology of dengue in Malaysia: a preliminary report. J. Med. Entomol. 2, 203—208 (1965).
Rudnick, A. in Proceedings of the International Conference on Dengue/DHF (eds Pang, T. & Pathmanathan, R.) 7 (University of Malaysia Press, Kuala Lumpur, 1984).
Gubler, D. J. in Dengue and Dengue Hemorrhagic Fever (eds Gubler, D. J. & Kuno, G.) 1—22 (CAB International, New York, 1997).
Rico-Hesse, R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 174, 479—493 (1990).
Holmes, E. C. & Twiddy, S. S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 3, 19—28 (2003).
Harrington, L. C., Edman, J. D. & Scott, T. W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 38, 411—422 (2001).
Weaver, S. C., Coffey, L. L., Nussenzveig, R., Ortiz, D. & Smith, D. in Microbe—Vector Interactions in Vector-borne Diseases (eds Gillespie, S. H., Smith, G. L. & Osbourn, A.) 139—180 (Cambridge Univ. Press, Cambridge, 2004).
Tabachnick, W. J. & Powell, J. R. A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet. Res. 34, 215—229 (1979).
Black, W. C. et al. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 33, 379—88 (2002).
Moncayo, A. C. et al. Emergence of epidemic dengue through the adaptation of sylvatic progenitor viruses to anthropophilic mosquito vectors. Emerg. Infect. Dis. (in the press).
Guirakhoo, F. et al. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever—dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298, 146—159 (2002).
Weaver, S. C., Rico-Hesse, R. & Scott, T. W. Genetic diversity and slow rates of evolution in New World alphaviruses. Curr. Top. Microbiol. Immunol. 176, 99—117 (1992).
Weaver, S. C., Brault, A. C., Kang, W. & Holland, J. J. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J. Virol. 73, 4316—4326 (1999).
Cooper, L. A. & Scott, T. W. Differential evolution of eastern equine encephalitis virus populations in response to host cell type. Genetics 157, 1403—1412 (2001).
Novella, I. S., Hershey, C. L., Escarmis, C., Domingo, E. & Holland, J. J. Lack of evolutionary stasis during alternating replication of an arbovirus in insect and mammalian cells. J. Mol. Biol. 287, 459—465 (1999).
Brault, A. C. Genetic Analysis of Epizootic Venezuelan Equine Encephalitis Virus Emergence Mechanisms. Thesis, Univ. Texas Medical Branch, (2001).
Byrnes, A. P. & Griffin, D. E. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72, 7349—7356 (1998).
Klimstra, W. B., Ryman, K. D. & Johnston, R. E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72, 7357—7366 (1998).
Weaver, S. C. et al. Genetic determinants of Venezuelan equine encephalitis emergence. Arch. Virol. Suppl. 18, 43—64 (2004).
Weaver, S. C., Ferro, C., Barrera, R., Boshell, J. & Navarro, J. C. Venezuelan equine encephalitis. Annu. Rev. Entomol. 49, 141—174 (2004).
Acknowledgements
S.C.W. is suppported by the National Institutes of Health. A.D.T.B. is supported by the State of Texas Higher Education Coordinating Board and the Centers for Diseases Control.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
Infectious Disease Database
FURTHER INFORMATION
Glossary
- EPIZOOTIC
-
Higher than average amplification, or occurrence, of a disease or pathogen in non-human animals.
- STRAINS
-
Individual isolates or variants of a virus.
- VIRAEMIA
-
Presence of virus in the bloodstream — usually essential for transmission of arboviruses by arthropod vectors.
- SYLVATIC
-
Occurring in forest habitats.
- ENZOOTIC
-
A disease or maintenance transmission cycle occurring continuously among non-human animals in a particular region or locality.
- SUBTYPE
-
A distinct virus variant that can be antigenically distinguished from other closely related variants.
- SYMPATRIC
-
Having overlapping geographical distributions.
- ZOONOTIC
-
Pathogens or diseases that normally circulate among non-human animals but that can be transmitted to humans.
- OLD WORLD
-
Those parts of the world known to Europeans before the voyages of Christopher Columbus — Europe, Asia and Africa. The New World refers to the American continents.
- PERIDOMESTIC
-
In, and around, human habitations.
Rights and permissions
About this article
Cite this article
Weaver, S., Barrett, A. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol 2, 789–801 (2004). https://doi.org/10.1038/nrmicro1006
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1006