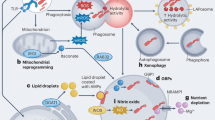
Key Points
-
The mechanisms by which phagocytes kill microorganisms are still not completely understood. Phagocyte-derived reactive oxygen species (ROS) and reactive nitrogen species (RNS) are undoubtedly important molecules in this process. Here, Ferric Fang reviews the biology of these molecules, with particular emphasis on aspects of their biology that remain controversial.
-
ROS and RNS are important molecules in host innate immunity. The author briefly reviews the diseases that occur when these molecules cannot be produced, including the roles of ROS in chronic granulomatous disease (CGD); RNS and inducible nitric oxide synthase (iNOS) promoter polymorphisms; and deficiencies in cytokine production or responses.
-
The NADPH phagocyte oxidase (phox)-mediated generation of ROS and the iNOS-mediated generation of RNS are described. The range of ROS and RNS species that are produced, and their cellular target molecules are also discussed.
-
Microorganisms can sometimes overcome the production of ROS and RNS to avoid being killed. The strategies that are used are reviewed, including evasion of ROS and RNS, suppression of ROS and RNS production, enzymic detoxification of reactive species, scavenging of the species to remove them, iron sequestration, stress responses and repairing the damage that the molecules cause.
-
Controversies in this field include the mechanisms by which nitric oxide is produced by macrophages, the roles of myeloperoxidase and xanthine oxidase, the importance of vesicular transport, the synergy between ROS and RNS, the synergy between ROS and proteases, and the generation of ROS by antibodies — all of which are discussed here.
Abstract
Phagocyte-derived reactive oxygen and nitrogen species are of crucial importance for host resistance to microbial pathogens. Decades of research have provided a detailed understanding of the regulation, generation and actions of these molecular mediators, as well as their roles in resisting infection. However, differences of opinion remain with regard to their host specificity, cell biology, sources and interactions with one another or with myeloperoxidase and granule proteases. More than a century after Metchnikoff first described phagocytosis, and more than four decades after the discovery of the burst of oxygen consumption that is associated with microbial killing, the seemingly elementary question of how phagocytes inhibit, kill and degrade microorganisms remains controversial. This review updates the reader on these concepts and the topical questions in the field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ambruso, D. R. et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl Acad. Sci. USA 97, 4654–4659 (2000).
Gray, G. R. et al. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet 2, 530–534 (1973).
Pollock, J. D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature Genet. 9, 202–209 (1995).
Jackson, S. H., Gallin, J. I. & Holland, S. M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182, 751–758 (1995). Presents a model of X-linked deficiency of the NADPH phagocyte oxidase.
The, International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon-γ to prevent infection in chronic granulomatous disease. N. Engl. J. Med. 324, 509–516 (1991).
Jackson, S. H. et al. IFN-γ is effective in reducing infections in the mouse model of chronic granulomatous disease (CGD). J. Interferon Cytokine Res. 21, 567–573 (2001).
Muhlebach, T. J. et al. Treatment of patients with chronic granulomatous disease with recombinant human interferon-γ does not improve neutrophil oxidative metabolism, cytochrome b558 content or levels of four anti-microbial proteins. Clin. Exp. Immunol. 88, 203–206 (1992).
Levesque, M. C. et al. Nitric oxide synthase type 2 promoter polymorphisms, nitric oxide production, and disease severity in Tanzanian children with malaria. J. Infect. Dis. 180, 1994–2002 (1999). Provides the first genetic evidence of the role of iNOS in human resistance to infection.
Hobbs, M. R. et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360, 1468–1475 (2002).
Kun, J. F. et al. Nitric oxide synthase 2 (Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 184, 330–336 (2001).
Lopansri, B. K. et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361, 676–678 (2003).
MacMicking, J. D. et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 (1995).
Nathan, C. & Shiloh, M. U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl Acad. Sci. USA 97, 8841–8848 (2000).
Mastroeni, P. et al. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192, 237–248 (2000).
Vazquez-Torres, A., Jones-Carson, J., Mastroeni, P., Ischiropoulos, H. & Fang, F. C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192, 227–236 (2000). References 14 and 15 describe the sequential contributions of NADPH phagocyte oxidase and iNOS in host resistance to Salmonella infection.
Shiloh, M. U. et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38 (1999). Shows that NADPH oxidase and NO˙ synthase might compensate for one another as mice that are deficient in both develop spontaneous infections with commensal flora.
Voskuil, M. I. et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 (2003).
Stenger, S., Donhauser, N., Thuring, H., Rollinghoff, M. & Bogdan, C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183, 1501–1514 (1996). Shows a clear role for NO˙ in the maintenance of latent infection.
Scanga, C. A. et al. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67, 4531–4538 (1999).
Choi, H. S., Rai, P. R., Chu, H. W., Cool, C. & Chan, E. D. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166, 178–186 (2002).
Qadoumi, M., Becker, I., Donhauser, N., Rollinghoff, M. & Bogdan, C. Expression of inducible nitric oxide synthase in skin lesions of patients with American cutaneous leishmaniasis. Infect. Immun. 70, 4638–4642 (2002).
Blos, M. et al. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur. J. Immunol. 33, 1224–1234 (2003).
Mastroeni, P. et al. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of γ-interferon and macrophage activation. Infect. Immun. 66, 4767–4776 (1998).
Xing, Z., Zganiacz, A. & Santosuosso, M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-γ induction. J. Leukoc. Biol. 68, 897–902 (2000).
de Jong, R. et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280, 1435–1438 (1998).
Morahan, G. et al. A promoter polymorphism in the gene encoding interleukin-12 p40 (IL12B) is associated with mortality from cerebral malaria and with reduced nitric oxide production. Genes Immun. 3, 414–418 (2002).
Babior, B. M., Lambeth, J. D. & Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397, 342–344 (2002). An up-to-date mini-review of NADPH oxidase.
Vignais, P. V. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol. Life Sci. 59, 1428–1459 (2002).
Cadwallader, K. A. et al. Regulation of phosphatidylinositol 3-kinase activity and phosphatidylinositol 3,4,5-trisphosphate accumulation by neutrophil priming agents. J. Immunol. 169, 3336–3344 (2002).
Ellson, C. D. et al. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox). Nature Cell Biol. 3, 679–682 (2001).
Cassatella, M. A. et al. Molecular basis of interferon-γ and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J. Biol. Chem. 265, 20241–20246 (1990).
Dinauer, M. C. Regulation of neutrophil function by Rac GTPases. Curr. Opin. Hematol. 10, 8–15 (2003).
Zhao, X., Carnevale, K. A. & Cathcart, M. K. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J. Biol. Chem. 278, 40788–40792 (2003).
Bokoch, G. M., Quilliam, L. A., Bohl, B. P., Jesaitis, A. J. & Quinn, M. T. Inhibition of Rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of Rap1A. Science 254, 1794–1796 (1991).
DeCoursey, T. E., Morgan, D. & Cherny, V. V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534 (2003).
Sbarra, A. J. & Karnovsky, M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 234, 1355–1362 (1959).
Hibbs, J. B. Jr. Infection and nitric oxide. J. Infect. Dis. 185 (Suppl. 1), S9–S17 (2002).
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001). The authors propose that thiol nitrosylation is a model for redox-based signal transduction.
Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Bogdan, C., Rollinghoff, M. & Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 173, 17–26 (2000).
Benjamin, N. & Dykhuizen, R. in Nitric Oxide and Infection (ed. Fang, F. C.) 215–230 (Kluwer Academic/Plenum Publishers, New York, 1999).
Stuehr, D. J. Mammalian nitric oxide synthases. Biochim Biophys. Acta 1411, 217–230 (1999).
Stuehr, D. J. & Marletta, M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl Acad. Sci. USA 82, 7738–7742 (1985).
Xie, Q. W., Kashiwabara, Y. & Nathan, C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269, 4705–4708 (1994).
Kamijo, R. et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263, 1612–1615 (1994).
Taylor, B. S. & Geller, D. A. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock 13, 413–424 (2000). Discusses the fundamental differences between human and murine iNOS promoters.
Vazquez-Torres, A. et al. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 172, 6202–6208 (2004).
Kolodziejski, P. J., Musial, A., Koo, J. S. & Eissa, N. T. Ubiquitination of inducible nitric oxide synthase is required for its degradation. Proc. Natl Acad. Sci. USA 99, 12315–12320 (2002).
El-Gayar, S., Thuring-Nahler, H., Pfeilschifter, J., Rollinghoff, M. & Bogdan, C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J. Immunol. 171, 4561–4568 (2003).
Schoedon, G. et al. Regulation of the L-arginine-dependent and tetrahydrobiopterin-dependent biosynthesis of nitric oxide in murine macrophages. Eur. J. Biochem. 213, 833–839 (1993).
Nathan, C. & Xie, Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell 78, 915–918 (1994).
Gaston, B. & Stamler, J. S. in Nitric Oxide and Infection (ed. Fang, F. C.) 37–55 (Kluwer Academic/Plenum Publishers, New York, 1999).
De Groote, M. A. & Fang, F. C. in Nitric Oxide and Infection (ed. Fang, F. C.) 231–261 (Kluwer Academic/Plenum Publishers, New York, 1999).
Imlay, J. A. & Linn, S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 166, 519–527 (1986). Shows that the mechanism of bacterial killing by H 2 O 2 is concentration dependent.
Imlay, J. A. & Linn, S. DNA damage and oxygen radical toxicity. Science 240, 1302–1309 (1988).
McCormick, M. L., Buettner, G. R. & Britigan, B. E. Endogenous superoxide dismutase levels regulate iron-dependent hydroxyl radical formation in Escherichia coli exposed to hydrogen peroxide. J. Bacteriol. 180, 622–625 (1998).
Tamarit, J., Cabiscol, E. & Ros, J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J. Biol. Chem. 273, 3027–3032 (1998).
Shohet, S. B., Pitt, J., Baehner, R. L. & Poplack, D. G. Lipid peroxidation in the killing of phagocytized pneumococci. Infect. Immun. 10, 1321–1328 (1974).
Imlay, J. A. Pathways of oxidative damage. Annu. Rev. Microbiol. 57, 395–418 (2003).
Stadler, N., Hofer, M. & Sigler, K. Mechanisms of Saccharomyces cerevisiae PMA1 H+-ATPase inactivation by Fe2+, H2O2 and Fenton reagents. Free Radic. Res. 35, 643–653 (2001).
De Groote, M. A. et al. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl Acad. Sci. USA 92, 6399–6403 (1995).
Schapiro, J. M., Libby, S. J. & Fang, F. C. Inhibition of bacterial DNA replication by zinc mobilization during nitrosative stress. Proc. Natl Acad. Sci. USA 100, 8496–8501 (2003). Indicates that zinc metalloproteins that are involved in DNA replication are a crucial target of nitrogen oxides.
Pacelli, R. et al. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 182, 1469–1479 (1995).
Stevanin, T. M. et al. Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bo′ or bd, from nitric oxide. J. Biol. Chem. 275, 35868–35875 (2000). Describe how NO˙ inhibits bacterial respiration, which is antagonized by a bacterial flavohaemoglobin that converts NO˙ to nitrate.
Shi, L., Jung, Y. J., Tyagi, S., Gennaro, M. L. & North, R. J. Expression of TH1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl Acad. Sci. USA 100, 241–246 (2003).
Lepoivre, M., Fieschi, F., Coves, J., Thelander, L. & Fontecave, M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem. Biophys. Res. Commun. 179, 442–448 (1991).
Woodmansee, A. N. & Imlay, J. A. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol. Microbiol. 49, 11–22 (2003).
Flint, D. H., Tuminello, J. F. & Emptage, M. H. The inactivation of Fe–S clusters containing hydrolyases by superoxide. J. Biol. Chem. 268, 22369–22376 (1993).
Keyer, K. & Imlay, J. A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl Acad. Sci. USA 93, 13635–13640 (1996).
Wink, D. A. et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 254, 1001–1003 (1991).
Burney, S., Caulfield, J. L., Niles, J. C., Wishnok, J. S. & Tannenbaum, S. R. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 424, 37–49 (1999).
Spek, E. J. et al. Recombinational repair is critical for survival of Escherichia coli exposed to nitric oxide. J. Bacteriol. 183, 131–138 (2001).
Evans, T. J. et al. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl Acad. Sci. USA 93, 9553–9558 (1996).
Miller, R. A. & Britigan, B. E. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10, 1–18 (1997). Reviews some of the many examples of bacterial oxidative-stress resistance mechanisms that promote virulence.
Celli, J. & Finlay, B. B. Bacterial avoidance of phagocytosis. Trends Microbiol. 10, 232–237 (2002).
Black, D. S. & Bliska, J. B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16, 2730–2744 (1997).
Rosqvist, R., Forsberg, A. & Wolf-Watz, H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59, 4562–4569 (1991).
Vazquez-Torres, A. et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287, 1655–1658 (2000). Shows that Salmonella translocates proteins into the macrophage cytosol, which seems to interfere with vesicular transport.
Chakravortty, D., Hansen-Wester, I. & Hensel, M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195, 1155–1166 (2002).
Cowley, S. C., Myltseva, S. V. & Nano, F. E. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol. Microbiol. 20, 867–874 (1996).
Bliska, J. B. & Black, D. S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63, 681–685 (1995).
Pearson, R. D., Symes, P., Conboy, M., Weiss, A. A. & Hewlett, E. L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J. Immunol. 139, 2749–2754 (1987).
Prada, J., Malinowski, J., Muller, S., Bienzle, U. & Kremsner, P. G. Effects of Plasmodium vinckei hemozoin on the production of oxygen radicals and nitrogen oxides in murine macrophages. Am. J. Trop. Med. Hyg. 54, 620–624 (1996).
Choi, K. S. & Dumler, J. S. Early induction and late abrogation of respiratory burst in A. phagocytophilum-infected neutrophils. Ann. NY Acad. Sci. 990, 488–493 (2003).
Mott, J., Rikihisa, Y. & Tsunawaki, S. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70, 1359–1366 (2002).
Carlyon, J. A., Chan, W. T., Galan, J., Roos, D. & Fikrig, E. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 169, 7009–7018 (2002).
Saura, M. et al. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity 10, 21–28 (1999).
Colasanti, M., Persichini, T., Venturini, G. & Ascenzi, P. S-nitrosylation of viral proteins: molecular bases for antiviral effect of nitric oxide. IUBMB Life 48, 25–31 (1999).
Cao, W., Bao, C. & Lowenstein, C. J. Inducible nitric oxide synthase expression inhibition by adenovirus E1A. Proc. Natl Acad. Sci. USA 100, 7773–7778 (2003).
Seyler, R. W. Jr, Olson, J. W. & Maier, R. J. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect. Immun. 69, 4034–4040 (2001).
Fang, F. C. et al. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl Acad. Sci. USA 96, 7502–7507 (1999).
Wilson, T. M., de Lisle, G. W. & Collins, D. M. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol. Microbiol. 15, 1009–1015 (1995).
Bishai, W. R., Howard, N. S., Winkelstein, J. A. & Smith, H. O. Characterization and virulence analysis of catalase mutants of Haemophilus influenzae. Infect. Immun. 62, 4855–4860 (1994).
Poole, R. K. & Hughes, M. N. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36, 775–783 (2000).
Pathania, R., Navani, N. K., Gardner, A. M., Gardner, P. R. & Dikshit, K. L. Nitric oxide scavenging and detoxification by the Mycobacterium tuberculosis haemoglobin, HbN in Escherichia coli. Mol. Microbiol. 45, 1303–1314 (2002).
Gardner, A. M., Helmick, R. A. & Gardner, P. R. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J. Biol. Chem. 277, 8172–8177 (2002).
Liu, L. et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410, 490–494 (2001).
Bryk, R., Griffin, P. & Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 (2000).
de Jesus-Berrios, M. et al. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13, 1963–1968 (2003).
Newton, G. L. et al. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178, 1990–1995 (1996).
Thomson, L., Denicola, A. & Radi, R. The trypanothione–thiol system in Trypanosoma cruzi as a key antioxidant mechanism against peroxynitrite-mediated cytotoxicity. Arch. Biochem. Biophys. 412, 55–64 (2003).
Brenot, A., King, K. Y., Janowiak, B., Griffith, O. & Caparon, M. G. Contribution of glutathione peroxidase to the virulence of Streptococcus pyogenes. Infect. Immun. 72, 408–413 (2004).
St John, G. et al. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl Acad. Sci. USA 98, 9901–9906 (2001).
De Groote, M. A., Testerman, T., Xu, Y., Stauffer, G. & Fang, F. C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science 272, 414–417 (1996). Shows that homocysteine antagonizes the actions of NO˙ in bacteria as well as in blood vessels.
van Guldener, C. & Stehouwer, C. D. Hyperhomocysteinemia, vascular pathology, and endothelial dysfunction. Semin. Thromb. Hemost. 26, 281–289 (2000).
Wang, Y., Aisen, P. & Casadevall, A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63, 3131–3136 (1995).
Chaturvedi, V., Wong, B. & Newman, S. L. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 156, 3836–3840 (1996).
Simpson, J. A., Smith, S. E. & Dean, R. T. Scavenging by alginate of free radicals released by macrophages. Free Radic. Biol. Med. 6, 347–353 (1989).
Andrews, S. C. Iron storage in bacteria. Adv. Microb. Physiol. 40, 281–351 (1998).
Almiron, M., Link, A. J., Furlong, D. & Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6, 2646–2654 (1992).
Hantke, K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4, 172–177 (2001).
Chen, C. Y. & Morse, S. A. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiology 145, 2967–2975 (1999).
Wai, S. N., Nakayama, K., Umene, K., Moriya, T. & Amako, K. Construction of a ferritin-deficient mutant of Campylobacter jejuni: contribution of ferritin to iron storage and protection against oxidative stress. Mol. Microbiol. 20, 1127–1134 (1996).
Halsey, T. A., Vazquez-Torres, A., Gravdahl, D. J., Fang, F. C. & Libby, S. J. The ferritin-like Dps protein is required for Salmonella enterica serovar typhimurium oxidative stress resistance and virulence. Infect. Immun. 72, 1155–1158 (2004).
D'Autreaux, B., Touati, D., Bersch, B., Latour, J. M. & Michaud-Soret, I. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl Acad. Sci. USA 99, 16619–16624 (2002).
Lobysheva, I. I., Stupakova, M. V., Mikoyan, V. D., Vasilieva, S. V. & Vanin, A. F. Induction of the SOS DNA repair response in Escherichia coli by nitric oxide donating agents: dinitrosyl iron complexes with thiol-containing ligands and S-nitrosothiols. FEBS Lett. 454, 177–180 (1999).
Christman, M. F., Morgan, R. W., Jacobson, F. S. & Ames, B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41, 753–762 (1985).
Greenberg, J. T., Monach, P., Chou, J. H., Josephy, P. D. & Demple, B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl Acad. Sci. USA 87, 6181–6185 (1990).
Zheng, M., Aslund, F. & Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721 (1998).
Paget, M. S., Kang, J. G., Roe, J. H. & Buttner, M. J. σR, an RNA polymerase σ-factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2). EMBO J. 17, 5776–5782 (1998).
Helmann, J. D. et al. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185, 243–253 (2003).
Boylan, J. A., Posey, J. E. & Gherardini, F. C. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl Acad. Sci. USA 100, 11684–11689 (2003).
Sak, B. D., Eisenstark, A. & Touati, D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc. Natl Acad. Sci. USA 86, 3271–3275 (1989).
Mukhopadhyay, P., Zheng, M., Bedzyk, L. A., LaRossa, R. A. & Storz, G. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl Acad. Sci. USA 101, 745–750 (2004). The authors argue that, despite some overlap, there seem to be important differences in the microbial transcriptional responses to oxidative and nitrosative stress.
Cruz-Ramos, H. et al. NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21, 3235–3244 (2002).
Zumft, W. G. Nitric oxide signaling and NO dependent transcriptional control in bacterial denitrification by members of the FNR-CRP regulator family. J. Mol. Microbiol. Biotechnol. 4, 277–286 (2002).
Suvarnapunya, A. E., Lagasse, H. A. & Stein, M. A. The role of DNA base excision repair in the pathogenesis of Salmonella enterica serovar typhimurium. Mol. Microbiol. 48, 549–559 (2003).
Darwin, K. H., Ehrt, S., Gutierrez-Ramos, J. C., Weich, N. & Nathan, C. F. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302, 1963–1966 (2003).
Buchmeier, N. A., Lipps, C. J., So, M. Y. & Heffron, F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol. Microbiol. 7, 933–936 (1993).
O'Rourke, E. J. et al. Pathogen DNA as target for host-generated oxidative stress: role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proc. Natl Acad. Sci. USA 100, 2789–2794 (2003).
Buchmeier, N. A. et al. DNA repair is more important than catalase for Salmonella virulence in mice. J. Clin. Invest. 95, 1047–1053 (1995).
Brawn, M. K. & Fridovich, I. Increased superoxide radical production evokes inducible DNA repair in Escherichia coli. J. Biol. Chem. 260, 922–925 (1985).
Zheng, M. et al. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183, 4562–4570 (2001).
Gerschman, R., Gilbert, D. L., Nye, S. W., Dwyer, P. & Fenn, W. O. Oxygen poisoning and X-irradiation: a mechanism in common. Science 119, 623–626 (1954).
Schneemann, M. et al. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J. Infect. Dis. 167, 1358–1363 (1993).
Weinberg, J. B. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4, 557–591 (1998). Reviews compelling evidence for NO˙ production by human macrophages.
Wheeler, M. A. et al. Bacterial infection induces nitric oxide synthase in human neutrophils. J. Clin. Invest. 99, 110–116 (1997).
Schneemann, M. & Schoedon, G. Species differences in macrophage NO production are important. Nature Immunol. 3, 102 (2002).
Fang, F. C. & Vazquez-Torres, A. Nitric oxide production by human macrophages: there's NO doubt about it. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L941–L943 (2002).
Nicholson, S. et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183, 2293–2302 (1996).
Annane, D. et al. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet 355, 1143–1148 (2000).
Pham, T. N. et al. Elevated serum nitric oxide levels in patients with inflammatory arthritis associated with co-expression of inducible nitric oxide synthase and protein kinase C-η in peripheral blood monocyte-derived macrophages. J. Rheumatol. 30, 2529–2534 (2003).
Nauseef, W. M. & Malech, H. L. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood 67, 1504–1507 (1986).
Daugherty, A., Dunn, J. L., Rateri, D. L. & Heinecke, J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94, 437–444 (1994).
Foote, C. S., Goyne, T. E. & Lehrer, R. I. Assessment of chlorination by human neutrophils. Nature 301, 715–716 (1983).
Eiserich, J. P. et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391, 393–397 (1998).
Klebanoff, S. J. Iodination of bacteria: a bactericidal mechanism. J. Exp. Med. 126, 1063–1078 (1967).
Rosen, H. & Klebanoff, S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J. Exp. Med. 149, 27–39 (1979).
Rosen, H., Orman, J., Rakita, R. M., Michel, B. R. & VanDevanter, D. R. Loss of DNA–membrane interactions and cessation of DNA synthesis in myeloperoxidase-treated Escherichia coli. Proc. Natl Acad. Sci. USA 87, 10048–10052 (1990).
Thomas, E. L. & Aune, T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase–hydrogen peroxide–iodide antimicrobial system. Antimicrob. Agents Chemother. 13, 1006–1010 (1978).
Rakita, R. M., Michel, B. R. & Rosen, H. Myeloperoxidase-mediated inhibition of microbial respiration: damage to Escherichia coli ubiquinol oxidase. Biochemistry 28, 3031–3036 (1989).
Rosen, H., Crowley, J. R. & Heinecke, J. W. Human neutrophils use the myeloperoxidase–hydrogen peroxide–chloride system to chlorinate but not nitrate bacterial proteins during phagocytosis. J. Biol. Chem. 277, 30463–30468 (2002).
Chapman, A. L., Hampton, M. B., Senthilmohan, R., Winterbourn, C. C. & Kettle, A. J. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 277, 9757–9762 (2002).
Saito, M. et al. H2O2-nonproducing Streptococcus pyogenes strains: survival in stationary phase and virulence in chronic granulomatous disease. Microbiology 147, 2469–2477 (2001).
Kottilil, S., Malech, H. L., Gill, V. J. & Holland, S. M. Infections with Haemophilus species in chronic granulomatous disease: insights into the interaction of bacterial catalase and H2O2 production. Clin. Immunol. 106, 226–230 (2003).
Parry, M. F. et al. Myeloperoxidase deficiency: prevalence and clinical significance. Ann. Intern. Med. 95, 293–301 (1981). Shows that humans with MPO deficiency have little or no immunocompromise, in contrast to individuals with CGD.
Aratani, Y. et al. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun. 67, 1828–1836 (1999). Presents a description of an MPO-deficient mouse model.
Brennan, M. L. et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J. Clin. Invest. 107, 419–430 (2001).
Eiserich, J. P. et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296, 2391–2394 (2002).
Brennan, M. L. et al. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 349, 1595–1604 (2003).
Vorbach, C., Harrison, R. & Capecchi, M. R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 24, 512–517 (2003).
Takao, S. et al. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am. J. Physiol. 271, C1278–C1284 (1996).
Umezawa, K. et al. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect. Immun. 65, 2932–2940 (1997).
Segal, B. H. et al. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47phox−/− mouse model of chronic granulomatous disease. Infect. Immun. 68, 2374–2378 (2000).
Simmonds, H. A., Goday, A. & Morris, G. S. Superoxide radicals, immunodeficiency and xanthine oxidase activity: man is not a mouse! Clin. Sci. (Lond) 68, 561–565 (1985).
Aratani, Y. et al. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med. Mycol. 40, 557–563 (2002).
Miyakawa, H. et al. Effects of inducible nitric oxide synthase and xanthine oxidase inhibitors on SEB-induced interstitial pneumonia in mice. Eur. Respir. J. 19, 447–457 (2002).
Moorhouse, P. C., Grootveld, M., Halliwell, B., Quinlan, J. G. & Gutteridge, J. M. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 213, 23–28 (1987).
Frayha, R. A., Salti, I. S., Arnaout, A., Khatchadurian, A. & Uthman, S. M. Hereditary xanthinuria: report on three patients and short review of the literature. Nephron 19, 328–332 (1977).
Vorbach, C., Scriven, A. & Capecchi, M. R. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 16, 3223–3235 (2002).
Wang, J. et al. Serum xanthine oxidase: origin, regulation, and contribution to control of trypanosome parasitemia. Antioxid. Redox Signal. 4, 161–178 (2002).
Sengelov, H., Nielsen, M. H. & Borregaard, N. Separation of human neutrophil plasma membrane from intracellular vesicles containing alkaline phosphatase and NADPH oxidase activity by free flow electrophoresis. J. Biol. Chem. 267, 14912–14917 (1992).
Badwey, J. A. et al. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J. Cell. Physiol. 105, 541–545 (1980).
Ohno, Y., Hirai, K., Kanoh, T., Uchino, H. & Ogawa, K. Subcellular localization of H2O2 production in human neutrophils stimulated with particles and an effect of cytochalasin-B on the cells. Blood 60, 253–260 (1982).
Heyworth, P. G. et al. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558 . J. Clin. Invest. 87, 352–356 (1991).
Allen, L. A. et al. Transient association of the nicotinamide adenine dinucleotide phosphate oxidase subunits p47phox and p67phox with phagosomes in neutrophils from patients with X-linked chronic granulomatous disease. Blood 93, 3521–3530 (1999).
Kobayashi, T., Robinson, J. M. & Seguchi, H. Identification of intracellular sites of superoxide production in stimulated neutrophils. J. Cell Sci. 111, 81–91 (1998). Shows that active NADPH oxidase might assemble in vesicles that subsequently move to the plasma membrane or phagosomal compartments.
Seguchi, H. & Kobayashi, T. Study of NADPH oxidase-activated sites in human neutrophils. J. Electron Microsc. (Tokyo) 51, 87–91 (2002).
Lang, M. L. & Kerr, M. A. Neutrophil NADPH oxidase does not assemble on macropinocytic vacuole membranes. Immunol. Lett. 72, 1–6 (2000).
Kobayashi, T. et al. A simple approach for the analysis of intracellular movement of oxidant-producing intracellular compartments in living human neutrophils. Histochem. Cell Biol. 113, 251–257 (2000).
Johansson, A. et al. Different subcellular localization of cytochrome b and the dormant NADPH-oxidase in neutrophils and macrophages: effect on the production of reactive oxygen species during phagocytosis. Cell. Immunol. 161, 61–71 (1995).
Badwey, J. A. et al. Comparative biochemical and cytochemical studies on superoxide and peroxide in mouse macrophages. J. Cell. Physiol. 115, 208–216 (1983).
Calafat, J. et al. Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood 81, 3122–3129 (1993).
Teixeira, C. F., Azevedo, N. L., Carvalho, T. M., Fuentes, J. & Nagao, P. E. Cytochemical study of Streptococcus agalactiae and macrophage interaction. Microsc. Res. Tech. 54, 254–259 (2001).
Vazquez-Torres, A., Fantuzzi, G., Edwards, C. K., Dinarello, C. A. & Fang, F. C. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc. Natl Acad. Sci. USA 98, 2561–2565 (2001).
Vodovotz, Y., Russell, D., Xie, Q. W., Bogdan, C. & Nathan, C. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J. Immunol. 154, 2914–2925 (1995).
Fang, F. & Vazquez-Torres, A. Salmonella selectively stops traffic. Trends Microbiol. 10, 391–392 (2002).
Webb, J. L., Harvey, M. W., Holden, D. W. & Evans, T. J. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 69, 6391–6400 (2001).
Brunelli, L., Crow, J. P. & Beckman, J. S. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch. Biochem. Biophys. 316, 327–334 (1995).
Hickman-Davis, J., Gibbs-Erwin, J., Lindsey, J. R. & Matalon, S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl Acad. Sci. USA 96, 4953–4958 (1999).
Vazquez-Torres, A., Jones-Carson, J. & Balish, E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect. Immun. 64, 3127–3133 (1996).
Darrah, P. A., Hondalus, M. K., Chen, Q., Ischiropoulos, H. & Mosser, D. M. Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect. Immun. 68, 3587–3593 (2000).
De Groote, M. A. et al. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl Acad. Sci. USA 94, 13997–14001 (1997).
Reeves, E. P. et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291–297 (2002). This report proposes a new mechanism for the synergy between NADPH phagocyte oxidase and neutrophil granule proteases.
Tkalcevic, J. et al. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12, 201–210 (2000).
Ahluwalia, J. et al. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature 427, 853–858 (2004).
Roos, D. & Winterbourn, C. C. Immunology. Lethal weapons. Science 296, 669–671 (2002).
Staudinger, B. J., Oberdoerster, M. A., Lewis, P. J. & Rosen, H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J. Clin. Invest. 110, 1151–1163 (2002). Presents evidence that the bacteria in neutrophils experience oxidative stress and that their ability to respond is crucial for their intracellular survival.
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against Gram-negative bacterial sepsis. Nature Med. 4, 615–618 (1998).
MacIvor, D. M. et al. Normal neutrophil function in cathepsin G-deficient mice. Blood 94, 4282–4293 (1999).
Murray, H. W. & Cartelli, D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J. Clin. Invest. 72, 32–44 (1983).
Mandell, G. L. & Hook, E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J. Bacteriol. 100, 531–532 (1969).
Kim, Y. M., Hong, S. J., Billiar, T. R. & Simmons, R. L. Counterprotective effect of erythrocytes in experimental bacterial peritonitis is due to scavenging of nitric oxide and reactive oxygen intermediates. Infect. Immun. 64, 3074–3080 (1996).
Speert, D. P., Bond, M., Woodman, R. C. & Curnutte, J. T. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170, 1524–1531 (1994).
Dinauer, M. C., Gifford, M. A., Pech, N., Li, L. L. & Emshwiller, P. Variable correction of host defense following gene transfer and bone marrow transplantation in murine X-linked chronic granulomatous disease. Blood 97, 3738–3745 (2001).
Harrison, R. E., Touret, N. & Grinstein, S. Microbial killing: oxidants, proteases and ions. Curr. Biol. 12, R357–R359 (2002).
Wentworth, P. Jr et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 298, 2195–2199 (2002). Suggests that ozone is an antimicrobial mediator that is formed by antibodies.
Takeuchi, K. & Ibusuki, T. Quantitative determination of aqueous-phase ozone by chemiluminescence using indigo-5,5′-disulfonate. Anal. Chem. 61, 619–623 (1989).
Kettle, A. J., Clark, B. M. & Winterbourn, C. C. Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone. J. Biol. Chem. 279, 18521–18525 (2004).
Parren, P. W., Leusen, J. H. & van de Winkel, J. G. Antibody-catalyzed water oxidation: state-of-the-art immunity or ancient history? Trends Immunol. 24, 467–469 (2003).
Forman, H. J. & Torres, M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 166, S4–S8 (2002).
Kim, S. & Ponka, P. Role of nitric oxide in cellular iron metabolism. Biometals 16, 125–135 (2003).
Nathan, C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 111, 769–778 (2003).
Ehrt, S. et al. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194, 1123–1140 (2001). Describes the profound effects of NADPH oxidase and iNOS on gene expression.
Kobayashi, S. D. et al. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J. Immunol. 172, 636–643 (2004).
Cooper, A. M., Adams, L. B., Dalton, D. K., Appelberg, R. & Ehlers, S. IFN-γ and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 10, 221–226 (2002).
Alam, M. S. et al. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect. Immun. 70, 3130–3142 (2002).
Sadikot, R. T. et al. p47phox deficiency impairs NF-κB activation and host defense in Pseudomonas pneumonia. J. Immunol. 172, 1801–1808 (2004).
Huang, J. et al. The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc. Natl Acad. Sci. USA 99, 3914–3919 (2002).
van der Veen, R. C. et al. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J. Immunol. 164, 5177–5183 (2000).
Li, H. & Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 190, 244–254 (2000).
Fang, F. C. Nitric Oxide and Infection 517 (Kluwer Academic/Plenum Publishers, New York, 1999). A comprehensive examination of the many roles of NO˙ in infection.
Petros, A. et al. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc. Res. 28, 34–39 (1994).
Cobb, J. P. Use of nitric oxide synthase inhibitors to treat septic shock: the light has changed from yellow to red. Crit. Care Med. 27, 855–856 (1999). Argues that although initial clinical studies of NO˙ inhibition in sepsis have been disappointing, selective inhibitors await more extensive evaluation.
Akaike, T. et al. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl Acad. Sci. USA 93, 2448–2453 (1996). Shows that the inhibition of ROS/RNS-mediated tissue damage can reduce mortality in certain infections.
Adler, H. et al. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2). J. Exp. Med. 185, 1533–1540 (1997).
Davis, I. C. et al. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J. Virol. 76, 8347–8259 (2002).
Fujii, S., Akaike, T. & Maeda, H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology 256, 203–212 (1999).
Suntres, Z. E., Omri, A. & Shek, P. N. Pseudomonas aeruginosa-induced lung injury: role of oxidative stress. Microb. Pathog. 32, 27–34 (2002).
Auer, M., Pfister, L. A., Leppert, D., Tauber, M. G. & Leib, S. L. Effects of clinically used antioxidants in experimental pneumococcal meningitis. J. Infect. Dis. 182, 347–350 (2000).
de Gans, J. & van de Beek, D. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347, 1549–1556 (2002).
Umeki, S. & Soejima, R. Hydrocortisone inhibits the respiratory burst oxidase from human neutrophils in whole-cell and cell-free systems. Biochim Biophys. Acta 1052, 211–215 (1990).
Korhonen, R., Lahti, A., Hamalainen, M., Kankaanranta, H. & Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 62, 698–704 (2002).
Gao, X. P. et al. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 168, 3974–3982 (2002).
Morgenstern, D. E., Gifford, M. A., Li, L. L., Doerschuk, C. M. & Dinauer, M. C. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185, 207–218 (1997). Shows that NADPH oxidase might be important in the resolution of inflammatory lesions.
Weiss, J., Kao, L., Victor, M. & Elsbach, P. Respiratory burst facilitates the digestion of Escherichia coli killed by polymorphonuclear leukocytes. Infect. Immun. 55, 2142–2147 (1987).
Carson, M. J., Chadwick, D. L., Brubaker, C. A., Cleland, R. S. & Landing, B. H. Thirteen boys with progressive septic granulomatosis. Pediatrics 35, 405–412 (1965).
Pignatelli, B. et al. Helicobacter pylori eradication attenuates oxidative stress in human gastric mucosa. Am. J. Gastroenterol. 96, 1758–1766 (2001).
Blanchard, T. G., Yu, F., Hsieh, C. L. & Redline, R. W. Severe inflammation and reduced bacteria load in murine helicobacter infection caused by lack of phagocyte oxidase activity. J. Infect. Dis. 187, 1609–1615 (2003).
Laroux, F. S. et al. Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid. Redox Signal. 2, 391–396 (2000).
Anstey, N. M. et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184, 557–567 (1996).
Matsushita, K. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139–150 (2003).
Acknowledgements
The author is deeply grateful for the insight and suggestions provided by M. Dinauer, S. Holland, C. Lowenstein, C. Nathan, W. Nauseef, H. Rosen and A. Vazquez-Torres, as well as the members of the Fang laboratory. Space limitations prevented the inclusion of many noteworthy citations — in such instances, the author has cited a representative article or referred the reader to a relevant review. Research support was provided by the US National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Infectious Disease Information
OMIM
SwissProt
glucose-6-phosphate dehydrogenase
FURTHER INFORMATION
Glossary
- PATHOPHYSIOLOGICAL
-
Functional changes that are associated with, or result from, disease or injury.
- POLYMORPHONUCLEAR PHAGOCYTES
-
White blood cells with multi-lobed nuclei and cytoplasmic granules that are involved in inflammatory responses.
- PROINFLAMMATORY CYTOKINES
-
Secreted proteins with autocrine or paracrine action that regulate the inflammatory response. There are many types of cytokine, which elicit different cellular responses, including the control of cell proliferation and differentiation, the regulation of immune responses and haematopoiesis.
- AGONIST PEPTIDES
-
Peptides that mimic cognate antigen, which results in cellular activation.
- ELECTROGENIC
-
Generating an electrical potential across a membrane.
- DENDRITIC CELLS
-
'Professional' antigen-presenting cells that are found in the T-cell areas of lymphoid tissues and as minor cellular components in most tissues. They have a branched or dendritic morphology and are the most potent stimulators of T-cell responses.
- ISOFORMS
-
Forms of a protein with slightly different amino-acid sequences that often have diverse activities, functions and/or distributions.
- HYPOHALOUS
-
A compound in which a hydroxyl group is combined with a halogen atom.
- FENTON REACTION
-
The reduction of hydrogen peroxide by ferrous iron.
- PEROXIDATION
-
A type of reaction in which oxygen atoms are formed, which leads to the production of peroxides.
- MYELOPEROXIDASE
-
Peroxidase from neutrophils that takes part in the bactericidal activity of these cells. The name originates from the first isolation from the blood of patients with myeloid leukaemia.
- PROTEASOME
-
In eukaryotes the 26S proteasome is a large multisubunit protease complex that selectively degrades multi-ubiquitylated proteins. It contains a 20S particle that carries the catalytic activity and two regulatory 19S particles.
- IONOPHORE
-
Small hydrophobic molecules that dissolve in lipid bilayers and increase the permeability of membranes to ions.
Rights and permissions
About this article
Cite this article
Fang, F. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2, 820–832 (2004). https://doi.org/10.1038/nrmicro1004
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1004