Key Points
-
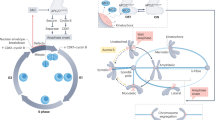
During mitosis, replicated sister chromatids attach to the mitotic spindle to facilitate their equal partitioning into two daughter cells.
-
Error-free chromosome segregation depends on the establishment of proper interactions between chromosomes and spindle microtubules, and on the delay of chromosome segregation, which is mediated by the activation of spindle assembly checkpoint (SAC), until all chromosomes have attached to the spindle. Both of these processes depend on the kinetochore, an assembly of proteins that connects centromeric DNA to spindle microtubules.
-
The KMN (kinetochore null protein 1 (KNL1)–missegregation 12 (MIS12)–nuclear division cycle 80 (NDC80) protein network constitutesan essential and conserved microtubule-binding activity at the kinetochore. Additionally, the KMN network is a platform for the recruitment of SAC proteins to the kinetochore, an essential step in the activation of checkpoint signalling.
-
Kinetochore–microtubule interactions are regulated by reversible protein phosphorylation, with essential contributions from kinases and phosphatases, and by protein–protein interactions. This ensures that proper spindle attachments persist until anaphase, whereas improper attachments are eliminated.
-
Phosphorylation-dependent protein–protein interactions between SAC proteins and the KMN network are important to coordinate the absence or presence of kinetochore-bound microtubules to regulate the activation or extinction of SAC signalling, respectively.
-
A complete understanding of how proper chromosome–spindle attachments are established and how their presence is relayed to the SAC to ensure error-free chromosome segregation will provide an integrated understanding of the molecular transactions at the kinetochore, together with its micromechanical properties, which function cooperatively to increase the fidelity of chromosome segregation.
Abstract
In eukaryotes, chromosome segregation during cell division is facilitated by the kinetochore, a multiprotein structure that is assembled on centromeric DNA. The kinetochore attaches chromosomes to spindle microtubules, modulates the stability of these attachments and relays the microtubule-binding status to the spindle assembly checkpoint (SAC), a cell cycle surveillance pathway that delays chromosome segregation in response to unattached kinetochores. Recent studies are shaping current thinking on how each of these kinetochore-centred processes is achieved, and how their integration ensures faithful chromosome segregation, focusing on the essential roles of kinase–phosphatase signalling and the microtubule-binding KMN protein network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McAinsh, A. D. & Meraldi, P. The CCAN complex: linking centromere specification to control of kinetochore–microtubule dynamics. Semin. Cell Dev. Biol. 22, 946–952 (2011).
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006). A seminal paper that identifies the KMN network as a conserved and essential microtubule-binding complex at the kinetochore.
Gordon, D. J., Resio, B. & Pellman, D. Causes and consequences of aneuploidy in cancer. Nature Rev. Genet. 13, 189–203 (2012).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Basu, J. et al. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146, 13–28 (1999).
Kitagawa, R. & Rose, A. M. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nature Cell Biol. 1, 514–521 (1999).
Dobles, M., Liberal, V., Scott, M. L., Benezra, R. & Sorger, P. K. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645 (2000).
Kalitsis, P., Earle, E., Fowler, K. J. & Choo, K. H. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 14, 2277–2282 (2000).
Gascoigne, K. E. & Taylor, S. S. How do anti-mitotic drugs kill cancer cells? J. Cell Sci. 122, 2579–2585 (2009).
Hayashi, M. T., Cesare, A. J., Fitzpatrick, J. A., Lazzerini-Denchi, E. & Karlseder, J. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nature Struct. Mol. Biol. 19, 387–394 (2012).
Orth, J. D., Loewer, A., Lahav, G. & Mitchison, T. J. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell 23, 567–576 (2012).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Foley, E. A., Maldonado, M. & Kapoor, T. M. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nature Cell Biol. 13, 1265–1271 (2011). Demonstrates for the first time that aphosphatasebalances the activity of kinetochore kinases to promote the formation of proper kinetochore–microtubule interactions.
Uetake, Y. & Sluder, G. Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr. Biol. 20, 1666–1671 (2010).
Cheeseman, I. M. et al. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255–2268 (2004).
Kline-Smith, S. L., Sandall, S. & Desai, A. Kinetochore–spindle microtubule interactions during mitosis. Curr. Opin. Cell Biol. 17, 35–46 (2005).
Ciferri, C. et al. Architecture of the human Ndc80–Hec1 complex, a critical constituent of the outer kinetochore. J. Biol. Chem. 280, 29088–29095 (2005).
Ciferri, C. et al. Implications for kinetochore–microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427–439 (2008).
Wei, R. R. et al. Structure of a central component of the yeast kinetochore: the Spc24p/Spc25p globular domain. Structure 14, 1003–1009 (2006).
Wei, R. R., Sorger, P. K. & Harrison, S. C. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl Acad. Sci. USA 102, 5363–5367 (2005).
Wei, R. R., Al-Bassam, J. & Harrison, S. C. The Ndc80/HEC1 complex is a contact point for kinetochore–microtubule attachment. Nature Struct. Mol. Biol. 14, 54–59 (2007).
Petrovic, A. et al. The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J. Cell Biol. 190, 835–852 (2010).
Bock, L. J. et al. Cnn1 inhibits the interactions between the KMN complexes of the yeast kinetochore. Nature Cell Biol. 6, 614–624 (2012).
Alushin, G. M. et al. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467, 805–810 (2010). High-resolutionstructure of the NDC80 complex in association with microtubules.
Sundin, L. J., Guimaraes, G. J. & Deluca, J. G. The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore–microtubule attachment in mitosis. Mol. Biol. Cell 22, 759–768 (2011).
Przewloka, M. R. et al. CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 21, 399–405 (2011).
Screpanti, E. et al. Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr. Biol. 21, 391–398 (2011).
Weiss, E. & Winey, M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132, 111–123 (1996).
Li, R. & Murray, A. W. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991).
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).
Hwang, L. H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).
Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Fang, G., Yu, H. & Kirschner, M. W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2, 163–171 (1998).
Kramer, E. R., Gieffers, C., Holzl, G., Hengstschlager, M. & Peters, J. M. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol. 8, 1207–1210 (1998).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Glotzer, M., Murray, A. W. & Kirschner, M. W. Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 (1991).
Yamamoto, A., Guacci, V. & Koshland, D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol. 133, 99–110 (1996).
Cohen-Fix, O., Peters, J. M., Kirschner, M. W. & Koshland, D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 10, 3081–3093 (1996).
Funabiki, H. et al. Cut2 proteolysis required for sister-chromatid seperation in fission yeast. Nature 381, 438–441 (1996).
Holloway, S. L., Glotzer, M., King, R. W. & Murray, A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73, 1393–1402 (1993).
King, R. W. et al. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 (1995).
Sudakin, V. et al. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185–197 (1995).
Schwab, M., Lutum, A. S. & Seufert, W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90, 683–693 (1997).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Chao, W. C., Kulkarni, K., Zhang, Z., Kong, E. H. & Barford, D. Structure of the mitotic checkpoint complex. Nature 484, 208–213 (2012).
da Fonseca, P. C. et al. Structures of APC/CCdh1 with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature 470, 274–278 (2011). References 45 and 46 reveal the structure of the APC/C and demonstrate how the mitotic checkpoint complex inhibits APC/C activity towards cyclin B and securin.
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Burton, J. L. & Solomon, M. J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21, 655–667 (2007).
Foe, I. T. et al. Ubiquitination of Cdc20 by the APC occurs through an intramolecular mechanism. Curr. Biol. 21, 1870–1877 (2011).
Foster, S. A. & Morgan, D. O. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol. Cell 47, 921–932 (2012).
Pan, J. & Chen, R. H. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 18, 1439–1451 (2004).
Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995).
Spencer, F. & Hieter, P. Centromere DNA mutations induce a mitotic delay in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 89, 8908–8912 (1992).
Wang, Y. & Burke, D. J. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 6838–6844 (1995).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Maciejowski, J. et al. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190, 89–100 (2010).
Maldonado, M. & Kapoor, T. M. Constitutive Mad1 targeting to kinetochores uncouples checkpoint signalling from chromosome biorientation. Nature Cell Biol. 13, 475–482 (2011).
Fraschini, R. et al. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 20, 6648–6659 (2001).
Kim, E. M. & Burke, D. J. DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore. PLoS Genet. 4, e1000015 (2008).
Malureanu, L. A. et al. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/CCdc20 in interphase. Dev. Cell 16, 118–131 (2009).
Meraldi, P., Draviam, V. M. & Sorger, P. K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7, 45–60 (2004).
De Antoni, A. et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005).
Luo, X. et al. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nature Struct. Biol. 7, 224–229 (2000).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002).
Luo, X. et al. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nature Struct. Mol. Biol. 11, 338–345 (2004).
Sironi, L. et al. Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).
Mapelli, M., Massimiliano, L., Santaguida, S. & Musacchio, A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell 131, 730–743 (2007).
Luo, X. & Yu, H. Protein metamorphosis: the two-state behavior of Mad2. Structure 16, 1616–1625 (2008).
Mapelli, M. & Musacchio, A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr. Opin. Struct. Biol. 17, 716–725 (2007).
Tang, Z., Shu, H., Oncel, D., Chen, S. & Yu, H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell 16, 387–397 (2004).
Zich, J. et al. Kinase activity of fission yeast Mph1 is required for Mad2 and Mad3 to stably bind the anaphase promoting complex. Curr. Biol. 22, 296–301 (2012).
King, E. M., Rachidi, N., Morrice, N., Hardwick, K. G. & Stark, M. J. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 21, 1163–1168 (2007).
Hewitt, L. et al. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1–C-Mad2 core complex. J. Cell Biol. 190, 25–34 (2010).
Kwiatkowski, N. et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nature Chem. Biol. 6, 359–368 (2010).
Santaguida, S., Tighe, A., D'Alise, A. M., Taylor, S. S. & Musacchio, A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190, 73–87 (2010).
He, X., Rines, D. R., Espelin, C. W. & Sorger, P. K. Molecular analysis of kinetochore–microtubule attachment in budding yeast. Cell 106, 195–206 (2001).
Janke, C. et al. The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 20, 777–791 (2001).
Martin-Lluesma, S., Stucke, V. M. & Nigg, E. A. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 (2002).
McCleland, M. L. et al. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101–114 (2003).
Wigge, P. A. & Kilmartin, J. V. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 152, 349–360 (2001).
London, N., Ceto, S., Ranish, J. A. & Biggins, S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 (2012).
Shepperd, L. A. et al. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr. Biol. 22, 891–899 (2012).
Yamagishi, Y., Yang, C. H., Tanno, Y. & Watanabe, Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nature Cell Biol. 14, 746–752 (2012). Identifies, together with references 81 and 82, KNL1 as a crucial substrate of MPS1 and demonstrates that phosphorylation of KNL1 is essential for SAC activity.
Rischitor, P. E., May, K. M. & Hardwick, K. G. Bub1 is a fission yeast kinetochore scaffold protein, and is sufficient to recruit other spindle checkpoint proteins to ectopic sites on chromosomes. PLoS ONE 2, e1342 (2007).
Vanoosthuyse, V., Valsdottir, R., Javerzat, J. P. & Hardwick, K. G. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell. Biol. 24, 9786–9801 (2004).
Johnson, V. L., Scott, M. I., Holt, S. V., Hussein, D. & Taylor, S. S. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 117, 1577–1589 (2004).
Kiyomitsu, T., Obuse, C. & Yanagida, M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell 13, 663–676 (2007).
Kops, G. J. et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169, 49–60 (2005).
Kiyomitsu, T., Murakami, H. & Yanagida, M. Protein interaction domain mapping of human kinetochore protein Blinkin reveals a consensus motif for binding of spindle assembly checkpoint proteins Bub1 and BubR1. Mol. Cell. Biol. 31, 998–1011 (2011).
Krenn, V., Wehenkel, A., Li, X., Santaguida, S. & Musacchio, A. Structural analysis reveals features of the spindle checkpoint kinase Bub1–kinetochore subunit Knl1 interaction. J. Cell Biol. 196, 451–467 (2012).
Lampson, M. A. & Kapoor, T. M. The human mitotic checkpoint protein BubR1 regulates chromosome–spindle attachments. Nature Cell Biol. 7, 93–98 (2005).
Meraldi, P. & Sorger, P. K. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24, 1621–1633 (2005).
Windecker, H., Langegger, M., Heinrich, S. & Hauf, S. Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin. EMBO Rep. 10, 1022–1028 (2009).
Magidson, V. et al. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 (2011). A must-read paper that combines high-resolution time-lapse imaging and electron microscopy to reveal the predominance of lateral kinetochore–microtubule interactions and the arrangement of chromosomes within the spindle in prometaphase.
Lampson, M. A., Renduchitala, K., Khodjakov, A. & Kapoor, T. M. Correcting improper chromosome–spindle attachments during cell division. Nature Cell Biol. 6, 232–237 (2004).
Pinsky, B. A., Kung, C., Shokat, K. M. & Biggins, S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nature Cell Biol. 8, 78–83 (2006).
Welburn, J. P. et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore–microtubule interface. Mol. Cell 38, 383–392 (2010). Identifies phosphorylation sites in the KMN network and reveals that combinatorial phosphorylation of the KMN network can produce gradedchanges in microtubule-binding activity.
Lampson, M. A. & Cheeseman, I. M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 21, 133–140 (2010).
Liu, D., Vader, G., Vromans, M. J., Lampson, M. A. & Lens, S. M. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science 323, 1350–1353 (2009). Reveals that centromere tension results in a spatial separation between Aurora B and its kinetochore substrates, contributing to the proper stabilization of correct attachments.
Salimian, K. J. et al. Feedback control in sensing chromosome biorientation by the Aurora B kinase. Curr. Biol. 21, 1158–1165 (2011).
Ruediger, R., Ruiz, J. & Walter, G. Human cancer-associated mutations in the Aα subunit of protein phosphatase 2A increase lung cancer incidence in Aα knock-in and knockout mice. Mol. Cell. Biol. 31, 3832–3844 (2011).
Westermarck, J. & Hahn, W. C. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 14, 152–160 (2008).
Cai, S., O'Connell, C. B., Khodjakov, A. & Walczak, C. E. Chromosome congression in the absence of kinetochore fibres. Nature Cell Biol. 11, 832–838 (2009).
Suijkerbuijk, S. J., Vleugel, M., Teixeira, A. & Kops, G. J. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore–microtubule attachments. Dev. Cell 23, 745–755 (2012).
Maresca, T. J. & Salmon, E. D. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 184, 373–381 (2009).
Uchida, K. S. et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 184, 383–390 (2009).
Liu, D. et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 (2010).
Posch, M. et al. Sds22 regulates Aurora B activity and microtubule–kinetochore interactions at mitosis. J. Cell Biol. 191, 61–74 (2010).
Warren, C. D. et al. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell 13, 3029–3041 (2002).
Kawashima, S. A., Yamagishi, Y., Honda, T., Ishiguro, K. & Watanabe, Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327, 172–177 (2010).
Coue, M., Lombillo, V. A. & McIntosh, J. R. Microtubule depolymerization promotes particle and chromosome movement in vitro. J. Cell Biol. 112, 1165–1175 (1991).
Miranda, J. J., De Wulf, P., Sorger, P. K. & Harrison, S. C. The yeast DASH complex forms closed rings on microtubules. Nature Struct. Mol. Biol. 12, 138–143 (2005).
Welburn, J. P. et al. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev. Cell 16, 374–385 (2009).
Westermann, S. et al. Formation of a dynamic kinetochore–microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Asbury, C. L., Gestaut, D. R., Powers, A. F., Franck, A. D. & Davis, T. N. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc. Natl Acad. Sci. USA 103, 9873–9878 (2006).
Westermann, S. et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440, 565–569 (2006).
Grishchuk, E. L. et al. Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc. Natl Acad. Sci. USA 105, 6918–6923 (2008).
Lampert, F., Hornung, P. & Westermann, S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J. Cell Biol. 189, 641–649 (2010).
Schmidt, J. C. et al. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev. Cell 23, 968–980 (2012).
Tien, J. F. et al. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by Aurora B. J. Cell Biol. 189, 713–723 (2010). Shows, together with references 118 and 119,that the Dam1 and SKA complexes target the NDC80 complex to microtubule tips and enhance its ability to establish persistent contacts.
Powers, A. F. et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 (2009).
Cheeseman, I. M., Enquist-Newman, M., Muller-Reichert, T., Drubin, D. G. & Barnes, G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 152, 197–212 (2001).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Gaitanos, T. N. et al. Stable kinetochore–microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 28, 1442–1452 (2009).
Hanisch, A., Sillje, H. H. & Nigg, E. A. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 25, 5504–5515 (2006).
Raaijmakers, J. A., Tanenbaum, M. E., Maia, A. F. & Medema, R. H. RAMA1 is a novel kinetochore protein involved in kinetochore–microtubule attachment. J. Cell Sci. 122, 2436–2445 (2009).
Daum, J. R. et al. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 19, 1467–1472 (2009).
Stevens, D., Gassmann, R., Oegema, K. & Desai, A. Uncoordinated loss of chromatid cohesion is a common outcome of extended metaphase arrest. PLoS ONE 6, e22969 (2011).
Chan, Y. W., Jeyaprakash, A. A., Nigg, E. A. & Santamaria, A. Aurora B controls kinetochore–microtubule attachments by inhibiting Ska complex–KMN network interaction. J. Cell Biol. 196, 563–571 (2012).
Gestaut, D. R. et al. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nature Cell Biol. 10, 407–414 (2008).
Varma, D. et al. Recruitment of the human Cdt1 replication licensing protein by the loop domain of Hec1 is required for stable kinetochore–microtubule attachment. Nature Cell Biol. 14, 593–603 (2012).
Hsu, K. S. & Toda, T. Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore–spindle attachment in fission yeast. Curr. Biol. 21, 214–220 (2011).
Nicklas, R. B. & Koch, C. A. Chromosome micromanipulation. III. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 43, 40–50 (1969).
Akiyoshi, B. et al. Tension directly stabilizes reconstituted kinetochore–microtubule attachments. Nature 468, 576–579 (2010). A groundbreaking paper that reported the first reconstitution of kinetochore–microtubule attachments in vitro.
Vanoosthuyse, V. & Hardwick, K. G. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 19, 1176–1181 (2009).
Pinsky, B. A., Nelson, C. R. & Biggins, S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 19, 1182–1187 (2009).
Rosenberg, J. S., Cross, F. R. & Funabiki, H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 21, 942–947 (2011).
Espeut, J., Cheerambathur, D. K., Krenning, L., Oegema, K. & Desai, A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J. Cell Biol. 196, 469–482 (2012). Reveals that microtubule association of KNL1 functions together with the recruitment of PP1 to silence the SAC at kinetochores.
Meadows, J. C. et al. Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev. Cell 20, 739–750 (2011). Shows, together with references135–138, that PP1 association with KNL1 is a key step in checkpoint extinction.
Gassmann, R. et al. Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 24, 957–971 (2010).
Howell, B. J. et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159–1172 (2001).
Mansfeld, J., Collin, P., Collins, M. O., Choudhary, J. S. & Pines, J. APC15 drives the turnover of MCC–CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nature Cell Biol. 13, 1234–1243 (2011).
Uzunova, K. et al. APC15 mediates CDC20 autoubiquitylation by APC/CMCC and disassembly of the mitotic checkpoint complex. Nature Struct. Mol. Biol. 19, 1116–1123 (2012).
Varetti, G., Guida, C., Santaguida, S., Chiroli, E. & Musacchio, A. Homeostatic control of mitotic arrest. Mol. Cell 44, 710–720 (2011).
Xia, G. et al. Conformation-specific binding of p31comet antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 23, 3133–3143 (2004).
Mapelli, M. et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 25, 1273–1284 (2006).
Westhorpe, F. G. Tighe, A., Lara-Gonzalez, P. & Taylor, S.S. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J. Cell Sci. 124, 3905–3916 (2011).
Teichner, A. et al. p31comet promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc. Natl Acad. Sci. USA 108, 3187–3192 (2011).
Wan, X. et al. Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684 (2009).
Joglekar, A. P., Bloom, K. & Salmon, E. D. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19, 694–699 (2009).
McEwen, B. F., Heagle, A. B., Cassels, G. O., Buttle, K. F. & Rieder, C. L. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 137, 1567–1580 (1997).
Guse, A., Carroll, C. W., Moree, B., Fuller, C. J. & Straight, A. F. In vitro centromere and kinetochore assembly on defined chromatin templates. Nature 477, 354–358 (2011).
Dumont, S., Salmon, E. D. & Mitchison, T. J. Deformations within moving kinetochores reveal different sites of active and passive force generation. Science 337, 355–358 (2012). References 149, 150 and 153 present technological breakthroughs in quantitative high-resolution imaging and provide nanometre-scale resolution of the architecture of eukaryotic kinetochores.
Fuller, B. G. et al. Midzone activation of Aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 (2008).
Acknowledgements
T.M.K. is grateful to the US National Institutes of Health (NIH) (GM98579). The authors apologize to their colleagues whose work could not be discussed owing to space limitations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cytokinesis
-
The division of the cytoplasm to generate two daughter cells. It typically follows chromosome segregation.
- Sister chromatids
-
Pairs of identical DNA sequences that are formed as a result of DNA replication. Sister chromatids are held together by cohesin complexes.
- Cohesin
-
A multiprotein complex that tethers replicated sister chromatids. Cohesin enables sister chromatids to resist separation even when exposed to microtubule-dependent pulling forces, possibly by encircling DNA. Proteolytic cleavage of cohesin by the enzyme separase allows chromosome segregation at anaphase.
- Centromeric DNA
-
A specialized chromosomal locus that is epigenetically defined by the presence of the histone H3 variant centromere-associated protein A (CENPA), which directs the assembly of the kinetochore.
- Constitutive centromeric-associated network
-
(CCAN). A conserved network of proteins that is assembled on centromeric DNA. It is required for the recruitment of most kinetochore proteins.
- Coiled-coil domains
-
Secondary structures composed of two or more -helices that entwine into a supercoil. These structures often mediate proteinprotein interactions and oligomerization.
- Calponin-homology domain
-
A protein module of ∼110 amino acids that is found in many cytoskeletal and signal transduction proteins.
- E-hook
-
Carboxy-terminal residues of -tubulin and -tubulin. The E-hook contains the acidic residues Glu and Asp.
- Microtubule plus ends
-
The ends of microtubule polymers with -tubulin subunits being exposed. They are more dynamic than the microtubule minus end (in which -tubulin is exposed). In cells, microtubule nucleation occurs only at the plus end.
- Cytoplasmic dynein
-
A multi-subunit, AAA+ ATPase, minus-end directed microtubule motor. During mitosis, it is essential for proper kinetochoremicrotubule attachments and spindle pole organization.
Rights and permissions
About this article
Cite this article
Foley, E., Kapoor, T. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 14, 25–37 (2013). https://doi.org/10.1038/nrm3494
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3494
This article is cited by
-
Distinct characteristics of the DNA damage response in mammalian oocytes
Experimental & Molecular Medicine (2024)
-
Principles and dynamics of spindle assembly checkpoint signalling
Nature Reviews Molecular Cell Biology (2023)
-
The Caenorhabditis elegans Shugoshin regulates TAC-1 in cilia
Scientific Reports (2023)
-
Pan-cancer analysis combined with experiments explores the oncogenic role of spindle apparatus coiled-coil protein 1 (SPDL1)
Cancer Cell International (2022)
-
Cell cycle control in cancer
Nature Reviews Molecular Cell Biology (2022)