Key Points
-
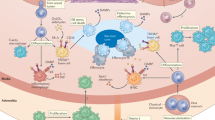
Atherosclerosis is a chronic inflammatory disease that is characterized by the continuous influx of mononuclear cells throughout all stages of plaque development, from early endothelial-cell dysfunction and foam-cell-rich fatty-streak lesions, through fibroproliferative progression and formation of a necrotic core and fibrous cap, to plaque destabilization and rupture.
-
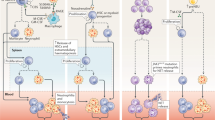
Neutrophils and their recruitment to evolving lesions contribute to atherogenesis and aneurysm formation, whereas protection conferred by the CXC-chemokine receptor 4 (CXCR4)– CXC ligand 12 (CXCL12) axis is due to the control of neutrophil homeostasis.
-
Protease and cytokine secretion by mast cells is involved in atheroprogression, plaque instability and aneurysm formation by promoting intraplaque haemorrhage apoptosis and subsequent leukocyte recruitment.
-
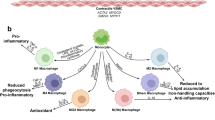
Recent evidence indicates that monocytes are crucial to the development of early atherogenesis. Two distinct subsets of monocytes are continually recruited during lesion progression, and independent roles for chemokine receptors in macrophage accumulation and lesion formation suggest that therapeutic strategies need to target both monocyte subsets and their multiple receptors.
-
Different subtypes of dendritic cell are present in atherosclerotic plaques, which can have pro-atherogenic effects through local or systemic priming of antigen-specific T cells or atheroprotective effects by inducing the deletion of the disease-causing T cells and by inducing regulatory T cells that produce the anti-inflammatory mediators interleukin-10 or transforming growth factor-β.
-
Although circulating smooth muscle progenitor cells can maintain plaque stability during advanced atherosclerosis, mobilization and recruitment of endothelial progenitor cells is associated with plaque growth, vascularization and destabilization, and incorporation of these cells at sites of endothelial-cell damage might support endothelial-cell regeneration.
Abstract
Chronic inflammation drives the development of atherosclerosis, and details regarding the involvement of different leukocyte subpopulations in the pathology of this disease have recently emerged. This Review highlights the surprising contribution of granulocyte subsets and mast cells to early atherogenesis and subsequent plaque instability, and describes the complex, double-edged role of monocyte, macrophage and dendritic-cell subsets through crosstalk with T cells and vascular progenitor cells. Improved understanding of the selective contributions of specific cell types to atherogenesis will pave the way for new targeted approaches to therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217 (2008). This study provides evidence for the recruitment and contribution of neutrophils to atherosclerotic plaques, which is controlled by a protective function of the CXCL12–CXCR4 axis.
van Leeuwen, M. et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler. Thromb. Vasc. Biol. 28, 84–89 (2008).
Bot, I. et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115, 2516–2525 (2007).
Sun, J. et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nature Med. 13, 719–724 (2007). References 4 and 5 provide important mechanistic insights on the role of mast cells and their secretory products in promoting atherogenesis and plaque instability.
Kostis, J. B., Turkevich, D. & Sharp, J. Association between leukocyte count and the presence and extent of coronary atherosclerosis as determined by coronary arteriography. Am. J. Cardiol. 53, 997–999 (1984).
Avanzas, P. et al. Multiple complex stenoses, high neutrophil count and C-reactive protein levels in patients with chronic stable angina. Atherosclerosis 175, 151–157 (2004).
Kawaguchi, H. et al. Band neutrophil count and the presence and severity of coronary atherosclerosis. Am. Heart J. 132, 9–12 (1996).
Naruko, T. et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 106, 2894–2900 (2002).
Larionov, S., Dedeck, O., Birkenmeier, G. & Thal, D. R. Expression of α2-macroglobulin, neutrophil elastase, and interleukin-1α differs in early-stage and late-stage atherosclerotic lesions in the arteries of the circle of Willis. Acta Neuropathol. 113, 33–43 (2007).
Eliason, J. L. et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 112, 232–240 (2005).
Zhang, R. et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286, 2136–2142 (2001).
Chevrier, I., Tregouet, D. A., Massonnet-Castel, S., Beaune, P. & Loriot, M. A. Myeloperoxidase genetic polymorphisms modulate human neutrophil enzyme activity: genetic determinants for atherosclerosis? Atherosclerosis 188, 150–154 (2006).
Hemdahl, A. L. et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 26, 136–142 (2006).
Galis, Z. S. & Khatri, J. J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 90, 251–262 (2002).
Soehnlein, O. et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112, 1461–1471 (2008).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Ait-Oufella, H. et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115, 2168–2177 (2007). This paper shows that the impaired capacity of macrophages for the clearance of apoptotic debris is due to genetic deletion of a crucial component that accelerates atherosclerosis.
Sugiyama, S. et al. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 1309–1314 (2004).
Kovanen, P. T., Kaartinen, M. & Paavonen, T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92, 1084–1088 (1995).
Kovanen, P. T. Mast cells: multipotent local effector cells in atherothrombosis. Immunol. Rev. 217, 105–122 (2007).
Hallgren, J. & Gurish, M. F. Pathways of murine mast cell development and trafficking: tracking the roots and routes of the mast cell. Immunol. Rev. 217, 8–18 (2007).
Constantinides, P. Mast cells and susceptibility to experimental atherosclerosis. Science 117, 505–506 (1953).
Lee-Rueckert, M. & Kovanen, P. T. Mast cell proteases: physiological tools to study functional significance of high density lipoproteins in the initiation of reverse cholesterol transport. Atherosclerosis 189, 8–18 (2006).
Jeziorska, M., McCollum, C. & Woolley, D. E. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J. Pathol. 182, 115–122 (1997).
Johnson, J. L., Jackson, C. L., Angelini, G. D. & George, S. J. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 18, 1707–1715 (1998).
Caughey, G. H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 217, 141–154 (2007).
Sun, J. et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J. Clin. Invest. 117, 3359–3368 (2007).
Haley, K. J. et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation 102, 2185–2189 (2000).
Bischoff, S. C., Krieger, M., Brunner, T. & Dahinden, C. A. Monocyte chemotactic protein 1 is a potent activator of human basophils. J. Exp. Med. 175, 1271–1275 (1992).
Watanabe, T. et al. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab. Invest. 53, 80–90 (1985).
Gown, A. M., Tsukada, T. & Ross, R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am. J. Pathol. 125, 191–207 (1986).
Braunersreuther, V. et al. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 27, 373–379 (2007).
Zernecke, A., Shagdarsuren, E. & Weber C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 19 June 2008 (doi:10.1161/ATVBAHA.107.161174).
Ylitalo, R., Oksala, O., Yla-Herttuala, S. & Ylitalo, P. Effects of clodronate (dichloromethylene bisphosphonate) on the development of experimental atherosclerosis in rabbits. J. Lab. Clin. Med. 123, 769–776 (1994).
Stoneman, V. et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 100, 884–893 (2007). This paper shows the differential effects of stage-specific depletion of monocytes and macrophages, which inhibited early atherogenesis and non-established plaques.
Swirski, F. K. et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007). This paper shows that monocyte subsets use different chemokine receptors for their recruitment to atherosclerotic plaques, which suggests that there are options for selective or combined antagonism.
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Weber, C. et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 67, 699–704 (2000).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Qiao, J. H. et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am. J. Pathol. 150, 1687–1699 (1997).
Saederup, N., Chan, L., Lira, S. A. & Charo, I. F. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117, 1642–1648 (2008).
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Dansky, H. M., Charlton, S. A., Harper, M. M. & Smith, J. D. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA 94, 4642–4646 (1997).
Zhou, X., Nicoletti, A., Elhage, R. & Hansson, G. K. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102, 2919–2922 (2000).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 6, 508–519 (2006).
Zhou, X., Paulsson, G., Stemme, S. & Hansson, G. K. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apoE-knockout mice. J. Clin. Invest. 101, 1717–1725 (1998).
Tellides, G. et al. Interferon-γ elicits arteriosclerosis in the absence of leukocytes. Nature 403, 207–211 (2000).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102, 1596–1601 (2005).
Mallat, Z. et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 85, e17–e24 (1999).
Furukawa, Y. et al. Interleukin-10 (IL-10) augments allograft arterial disease: paradoxical effects of IL-10 in vivo. Am. J. Pathol. 155, 1929–1939 (1999).
Rahmani, M., Cruz, R. P., Granville, D. J. & McManus, B. M. Allograft vasculopathy versus atherosclerosis. Circ. Res. 99, 801–815 (2006).
Schulte, S., Sukhova, G. K. & Libby, P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am. J. Pathol. 172, 1500–1508 (2008).
Henderson, E. L. et al. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 99, 96–104 (1999).
Taleb, S., Tedgui, A. & Mallat, Z. Regulatory T-cell immunity and its relevance to atherosclerosis. J. Intern. Med. 263, 489–499 (2008). This is a comprehensive review of the current knowledge of the regulatory T-cell response and the major cytokines that are involved in its modulation in the context of atherosclerosis.
Vanderlaan, P. A. & Reardon, C. A. Thematic review series: the immune system and atherogenesis. The unusual suspects: an overview of the minor leukocyte populations in atherosclerosis. J. Lipid Res. 46, 829–838 (2005).
Koch, A. E. et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am. J. Pathol. 137, 1199–1213 (1990).
Ramshaw, A. L. & Parums, D. V. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology 17, 543–552 (1990).
Walton, L. J., Powell, J. T. & Parums, D. V. Unrestricted usage of immunoglobulin heavy chain genes in B cells infiltrating the wall of atherosclerotic abdominal aortic aneurysms. Atherosclerosis 135, 65–71 (1997).
Watanabe, M. et al. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J. Atheroscler Thromb. 14, 325–331 (2007).
Zhou, X. & Hansson, G. K. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand. J. Immunol. 50, 25–30 (1999).
Moos, M. P. et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25, 2386–2391 (2005).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Caligiuri, G., Nicoletti, A., Poirier, B. & Hansson, G. K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109, 745–753 (2002). This study shows that spleen-associated immune protection against atherosclerosis in mice is conferred by B cells and is concomitant with increased antibody titres to oxLDL.
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007).
Millonig, G. et al. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler. Thromb. Vasc. Biol. 21, 503–508 (2001).
Jongstra-Bilen, J. et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 (2006).
Liu, P. et al. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 28, 243–250 (2008).
Bobryshev, Y. V. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur. Heart J. 26, 1700–1704 (2005).
Yilmaz, A. et al. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis 176, 101–110 (2004).
Yilmaz, A. et al. Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J. Am. Coll. Cardiol. 48, 70–80 (2006).
Shaposhnik, Z., Wang, X., Weinstein, M., Bennett, B. J. & Lusis, A. J. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 27, 621–627 (2007).
Han, J. W. et al. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ. Res. 102, 546–553 (2008).
Alderman, C. J. et al. Effects of oxidised low density lipoprotein on dendritic cells: a possible immunoregulatory component of the atherogenic micro-environment? Cardiovasc. Res. 55, 806–819 (2002).
Angeli, V. et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 (2004).
Shamshiev, A. T. et al. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8α-negative dendritic cells and protective Th1 type immunity. J. Exp. Med. 204, 441–452 (2007).
Niessner, A. et al. Synergistic proinflammatory effects of the antiviral cytokine interferon-α and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation 116, 2043–2052 (2007). This study shows that IFNα that is secreted by pDCs in atherosclerotic plaques stimulates IFNγ production by CD4+ T cells and sensitizes myeloid DCs to pathogen-derived TLR4 ligands to promote plaque destabilization.
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nature Immunol. 5, 1219–1226 (2004).
Niessner, A. et al. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-α. Circulation 114, 2482–2489 (2006).
Ochando, J. C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature Immunol. 7, 652–662 (2006).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Simper, D., Stalboerger, P. G., Panetta, C. J., Wang, S. & Caplice, N. M. Smooth muscle progenitor cells in human blood. Circulation 106, 1199–1204 (2002).
Urbich, C. & Dimmeler, S. Endothelial progenitor cells: characterization and role in vascular biology. Circ. Res. 95, 343–353 (2004).
Hristov, M. & Weber, C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J. Cell. Mol. Med. 8, 498–508 (2004).
Zengin, E. et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133, 1543–1551 (2006).
Peichev, M. et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95, 952–958 (2000).
Romagnani, P. et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ. Res. 97, 314–322 (2005).
Hristov, M. et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ. Res. 100, 590–597 (2007).
De Palma, M., Murdoch, C., Venneri, M. A., Naldini, L. & Lewis, C. E. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 28, 519–524 (2007).
Yoder, M. C. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 (2007).
Case, J. et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 35, 1109–1118 (2007).
Timmermans, F. et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler. Thromb. Vasc. Biol. 27, 1572–1579 (2007).
Sugiyama, S. et al. Characterization of smooth muscle-like cells in circulating human peripheral blood. Atherosclerosis 187, 351–362 (2006).
Zernecke, A. et al. SDF-1α/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 96, 784–791 (2005).
Sakihama, H. et al. Stromal cell-derived factor-1 and CXCR4 interaction is critical for development of transplant arteriosclerosis. Circulation 110, 2924–2930 (2004).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Med. 13, 587–596 (2007). This study describes how targeting the cytokine MIF as a dual chemokine-receptor agonist can inhibit both monocyte and T-cell recruitment, which then leads to plaque regression.
Haghighat, A., Weiss, D., Whalin, M. K., Cowan, D. P. & Taylor, W. R. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation 115, 2049–2054 (2007).
Werner, N. & Nickenig, G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler. Thromb. Vasc. Biol. 26, 257–266 (2006).
George, J. et al. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 25, 2636–2641 (2005). This study indicates that EPCs may promote atherosclerosis in mice, thereby introducing an important caveat to their clinical administration.
Foteinos, G., Hu, Y., Xiao, Q., Metzler, B. & Xu, Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation 117, 1856–1863 (2008).
Purhonen, S. et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl Acad. Sci. USA 105, 6620–6625 (2008).
Sata, M. et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nature Med. 8, 403–409 (2002).
Hillebrands, J. L., Klatter, F. A. & Rozing, J. Origin of vascular smooth muscle cells and the role of circulating stem cells in transplant arteriosclerosis. Arterioscler. Thromb. Vasc Biol. 23, 380–387 (2003).
Caplice, N. M. et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc. Natl Acad. Sci. USA 100, 4754–4759 (2003).
Bentzon, J. F., Sondergaard, C. S., Kassem, M. & Falk, E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation 116, 2053–2061 (2007).
Zoll, J. et al. Role of human smooth muscle cell progenitors in atherosclerotic plaque development and composition. Cardiovasc. Res. 77, 471–480 (2008). This study shows that infusion of SPCs in mice can limit atherosclerotic plaque development and promote plaque stability.
Uccelli, A., Pistoia, V. & Moretta, L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 28, 219–226 (2007).
Xu, Q. The role of stem cells in vein graft remodelling. Biochem. Soc. Trans. 35, 895–899 (2007).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Llodra, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA 101, 11779–11784 (2004). This study proposes that conversion of lesional monocyte-derived cells into migratory CD11c+ DC-like cells favours their egress from plaques to mediate regression.
Sozzani, S., Rusnati, M., Riboldi, E., Mitola, S. & Presta, M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 28, 385–392 (2007).
Menges, M. et al. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 195, 15–21 (2002).
Nilsson, J., Hansson, G. K. & Shah, P. K. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler. Thromb. Vasc. Biol. 25, 18–28 (2005).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Guven, H., Shepherd, R. M., Bach, R. G., Capoccia, B. J. & Link, D. C. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J. Am. Coll. Cardiol. 48, 1579–1587 (2006).
Xiao, Q. et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis — results from a large population-based study. PLoS ONE 2, e975 (2007).
Hristov, M. et al. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis 192, 413–420 (2007).
Acknowledgements
We would like to apologize to all colleagues whose contributions could not be cited because of space limitations. The authors are supported by grants from Deutsche Forschungsgemeinschaft (DFC-FOR809)
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Atherosclerosis
-
A chronic disorder of the arterial wall that starts by the formation of a fatty streak, which is an accumulation of lipid-loaded macrophages in the intima. This evolves into an atherosclerotic plaque by the migration and proliferation of smooth muscle cells, and the formation of a necrotic core and a fibrous cap with extracellular matrix components.
- Hyperlipidaemia
-
An excess of lipids, either triglycerides or cholesterol, in the blood. Lipids circulate as free or esterified entities in lipoprotein particles.
- Foam cell
-
A macrophage in the arterial wall that ingests oxidized low-density lipoprotein and assumes a foamy appearance. These cells secrete various substances involved in plaque growth.
- Intima
-
The innermost layer of an artery, consisting of loose connective tissue and covered by a monolayer of endothelium.
- Atherosclerotic plaque
-
A lesion that forms within the intima and media of large and medium arteries and that contains high levels of lipids, lipoproteins, activated macrophages, lipid-enriched foam cells and smooth muscle cells.
- Haemorrhage
-
A loss of blood or its components from the circulatory system.
- Fibrous cap
-
A structure composed of a dense collagen-rich extracellular matrix with occasional smooth muscle cells, macrophages and T cells that typically overlies the characteristic central lipid core of plaques.
- Aneurysm
-
The local dilatation of an artery that is caused by weakening of the artery wall. Some, but not all, aneurysms are caused by atherosclerosis.
- Atherogenesis
-
The early stages of atherosclerotic lesion formation, which results from the accumulation of monocytes and macrophages, and lipid-laden foam-cell formation in the intima.
- Regulatory T cell
-
A specialized CD4+ T cell that suppresses the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral self tolerance and are characterized by the expression of CD25 and the transcription factor forkhead box P3.
- B-1 cell
-
A minor population of B cells that is mainly present in the peritoneal and pleural cavities and that is characterized by a IgMhiIgDlowMAC1+B220lowCD23− phenotype. Their precursors develop in the fetal liver and omentum, and in adult mice the size of the B-1-cell population is kept constant owing to the self-renewing capacity of these cells. B-1 cells recognize self components, as well as common bacterial antigens, and they secrete antibodies that have low affinity and broad specificity.
- Coronary artery disease
-
A condition in which plaques build up inside the coronary arteries, which supply the heart muscle with oxygen-rich blood. The narrowing of coronary arteries (stenosis) by plaques reduces blood flow and can cause angina pectoris or, in the case of a plaque rupture and subsequent thrombosis, can lead to acute coronary syndromes, complete arterial occlusion and myocardial infarction.
- Myeloperoxidase
-
A peroxidase enzyme that is most highly expressed by neutrophils. It is a lysosomal protein that is stored in azurophilic granules of neutrophils. Myeloperoxidase produces hypochlorous acid from hydrogen peroxide and chloride ions during the respiratory burst in neutrophils.
- Elastase
-
A serine protease, similar to trypsin and chymotrypsin, that hydrolyses amides and esters. It is produced in the pancreas as an inactive zymogen, pro-elastase, and it is activated by trypsin and also by other enzymes in some leukocytes. Elastase specifically breaks down elastin in connective tissue.
- Adventitia
-
The outermost layer of an artery, which consists of connective tissue and harbours subsets of mononuclear cells, such as mast cells and resident vascular progenitor cells.
- Atheroprogression
-
The fibroproliferative progression and destabilization of plaques with fibrous-cap thinning, which eventually leads to erosion and rupture of the plaques.
- Autacoids
-
Locally produced lipid metabolites or hormones that have paracrine effects on vascular cells, such as smooth muscle cells, resulting in vasodilatation or vasoconstriction of the vessel. Some examples are eicosanoids, angiotensin, nitric oxide, kinins, histamine, serotonin or endothelin.
- Statins
-
A family of inhibitors of hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyses the conversion of HMG-CoA to L-mevalonate. These molecules are mainly used as cholesterol-lowering drugs, but they also have immunoregulatory and anti-inflammatory properties. L-Mevalonate and its metabolites are implicated in cholesterol synthesis and other intracellular pathways.
- Endothelial progenitor cell
-
(EPC). A progenitor cell that is derived from bone marrow, peripheral blood or vascular sources and is heterogeneous in terms of its origin and phenotype. EPCs express progenitor-cell markers (such as CD34) and endothelial-cell differentiation markers (such as CD31, VE-cadherin, von Willebrand factor and acetylated low-density lipoprotein–lectin-binding), have clonogenic capacity and form angiogenic structures.
- Smooth muscle progenitor cell
-
(SPC). A progenitor cell that can differentiate from bone-marrow, circulating or tissue-resident precursors, and expresses markers of the mesenchymal and smooth muscle cell lineage, such as endoglin, calponin, αSMA, SM-MHC, SM22 and PDGFRβ.
- Neointima
-
A smooth-muscle-cell-rich vascular hyperplasia, which forms in lieu of the intima following arterial injury or transplant arteriopathy and contributes to vessel stenosis.
- Vasa vasorum
-
A network of small nutrient vessels that are found in the normal adventitia and outer media of the artery wall and that can also give rise to microvessels in the plaque.
- Atherothrombosis
-
Following the rupture of unstable atherosclerotic plaques, thrombogenic material becomes exposed or released to mediate thrombus formation and eventually occlusion of an artery.
Rights and permissions
About this article
Cite this article
Weber, C., Zernecke, A. & Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol 8, 802–815 (2008). https://doi.org/10.1038/nri2415
Issue Date:
DOI: https://doi.org/10.1038/nri2415
This article is cited by
-
Executable models of immune signaling pathways in HIV-associated atherosclerosis
npj Systems Biology and Applications (2022)
-
SIRT6 mediates MRTF-A deacetylation in vascular endothelial cells to antagonize oxLDL-induced ICAM-1 transcription
Cell Death Discovery (2022)
-
The role of lymphangiogenesis in cardiovascular diseases and heart transplantation
Heart Failure Reviews (2022)
-
Accuracy of PET quantification in [68Ga]Ga-pentixafor PET/MR imaging of carotid plaques
Journal of Nuclear Cardiology (2022)
-
The involving progress of MSCs based therapy in atherosclerosis
Stem Cell Research & Therapy (2020)