Abstract
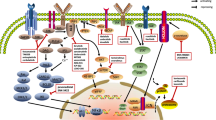
An improved understanding of the molecular biology of cancer cell growth and survival and the role of the microenvironment in supporting the survival of cancer cells, including lymphoma cells, has led to the identification of a number of potential therapeutic targets. Despite these advances, drug development for lymphoma remains slow, inefficient, and frequently unfocused. Future work should focus on identifying 'driver' molecular defects of oncogenic pathways that can be targeted therapeutically, discovering predictive biomarkers for treatment response, and prioritizing promising drugs to accelerate their approval. This Review summarizes the current development status of novel agents for lymphoma and discusses strategies to move the field forward.
Key Points
-
Lymphomas are heterogeneous group of malignancies with an estimated 74,000 new cases in 2009 in the USA
-
Several agents have been approved by the FDA for the treatment of relapsed non-Hodgkin lymphoma, but no drug has been approved for Hodgkin lymphoma in the past 30 years
-
Antibody–drug conjugates and small-molecule inhibitors that target well-defined oncogenic pathways are being evaluated for the treatment of lymphoma and have shown promising results
-
In the future, biomarker-driven clinical trials will be important for the development of personalized treatment strategies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal, A., Center, M. M., Ward, E. & Thun, M. J. Cancer occurrence. Methods Mol. Biol. 471, 3–29 (2009).
Zelenetz, A. D. et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's Lymphomas. J. Natl Compr. Canc. Netw. 8, 288–334 (2010).
Sridhara, R. et al. Review of oncology and hematology drug product approvals at the US Food and Drug Administration between July 2005 and December 2007. J. Natl Cancer Inst. 102, 230–243 (2010).
Cheson, B. D. & Leonard, J. P. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N. Engl. J. Med. 359, 613–626 (2008).
Coiffier, B. et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood 111, 1094–1100 (2008).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Hagenbeek, A. et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood 111, 5486–5495 (2008).
Hagenbeek, A. et al. Evaluation of ofatumumab, a novel human CD20 monoclonal antibody, as single agent therapy in rituximab-refractory follicular lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 935 (2009).
McLaughlin, P. et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 16, 2825–2833 (1998).
Maloney, D. G. Rituximab for follicular lymphoma. Curr. Hematol. Rep. 2, 13–22 (2003).
Leonard, J. P. et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J. Clin. Oncol. 21, 3051–3059 (2003).
Byrd, J. C. et al. Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin. Cancer Res. 13, 4448–4455 (2007).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008).
Baeuerle, P. A. & Reinhardt, C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 (2009).
Nagorsen, D. et al. Confirmation of safety, efficacy and response duration in non-Hodgkin lymphoma patients treated with 60 μg/m2/d of BiTE(R) antibody blinatumomab. Blood (ASH Annual Meeting Abstracts) 114, 2723 (2009).
Topp, M. S. et al. Report of a phase ii trial of single-agent BiTE(R) antibody blinatumomab in patients with minimal residual disease (MRD) positive B-precursor acute lymphoblastic leukemia (ALL). Blood (ASH Annual Meeting Abstracts) 114, 840 (2009).
Strauss, S. J. et al. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J. Clin. Oncol. 24, 3880–3886 (2006).
Micallef, I. N. et al. Final results of NCCTG N0489: Epratuzumab and rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy (ER-CHOP) in patients with previously untreated diffuse large B-cell lymphoma. J. Clin. Oncol. ASCO Meeting Abstracts 27 (Suppl.), 8508 (2009).
Younes, A. & Kadin, M. E. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J. Clin. Oncol. 21, 3526–3534 (2003).
Advani, R. et al. A phase 2 clinical trial of SGN-40 monotherapy in relapsed diffuse large B-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 112, 1000 (2008).
Advani, R. et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. J. Clin. Oncol. 27, 4371–4377 (2009).
Younes, A. et al. Results of a phase 2 trial of HGS-ETR1 (agonistic human monoclonal antibody to TRAIL receptor 1) in subjects with relapsed/refractory non-Hodgkin's lymphoma (NHL) (ETR1-01). Blood (ASH Annual Meeting Abstracts) 106, 489 (2005).
Wang, S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene 27, 6207–6215 (2008).
Bartlett, N. L. et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 111, 1848–1854 (2008).
Ansell, S. M. et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J. Clin. Oncol. 25, 2764–2769 (2007).
Blum, K. A. et al. Phase I study of an anti-CD30 Fc engineered humanized monoclonal antibody in Hodgkin lymphoma (HL) or anaplastic large cell lymphoma (ALCL) patients: Safety, pharmacokinetics (PK), immunogenicity, and efficacy. J. Clin. Oncol. ASCO Meeting Abstracts 27 (Suppl.), 8531 (2009).
Kreitman, R. J. et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J. Clin. Oncol. 23, 6719–6729 (2005).
Advani, A. et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J. Clin. Oncol. 28, 2085–2093 (2010).
Dang, N. H. et al. Anti-CD22 immunoconjugate inotuzumab ozogamicin (CMC-544) + rituximab: clinical activity including survival in patients with recurrent/refractory follicular or 'aggressive' lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 584 (2009).
Younes, A. et al. Phase I multi-dose escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous (IV) infusion every 3 weeks to patients with relapsed/ refractory B-cell non-hodgkin's lymphoma (NHL). Blood (ASH Annual Meeting Abstracts) 114, 585 (2009).
Aboukameel, A. et al. Superior anti-tumor activity of the CD19-directed immunotoxin, SAR3419 to rituximab in non-Hodgkin's xenograft animal models: preclinical evaluation. Blood (ASH Annual Meeting Abstracts) 110, 2339 (2007).
Oflazoglu, E., Kissler, K. M., Sievers, E. L., Grewal, I. S. & Gerber, H. P. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br. J. Haematol. 142, 69–73 (2008).
Younes, A. et al. Multiple complete responses in a phase 1 dose-escalation study of the antibody-drug conjugate SGN-35 in patients with relapsed or refractory CD30-positive lymphomas. Blood (ASH Annual Meeting Abstracts) 112, 1006 (2008).
Fanale, M. et al. The antibody-drug conjugate brentuximab vedotin (SGN-35) induced multiple objective responses in patients with relapsed or refractory CD30-positive lymphomas in a phase 1 weekly dosing study. Blood (ASH Annual Meeting Abstracts) 114, 2731 (2009).
Ihle, N. T. & Powis, G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol. Cancer Ther. 8, 1–9 (2009).
Franke, T. F. PI3K/Akt: getting it right matters. Oncogene 27, 6473–6488 (2008).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562 (2009).
Jaiswal, B. S. et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16, 463–474 (2009).
Berenjeno, I. M. & Vanhaesebroeck, B. PI3K regulatory subunits lose control in cancer. Cancer Cell 16, 449–450 (2009).
Georgakis, G. V., Yazbeck, V. Y., Li, Y. & Younes, A. Preclinical rationale for therapeutic targeting of mTOR by CC-I779 and rapamycin in Hodgkin lymphoma. J. Clin. Oncol. ASCO Meeting Abstracts 24 (Suppl.), 10070 (2006).
Jücker, M. et al. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin's lymphoma-derived cell line (CO). Leukemia 16, 894–901 (2002).
Morrison, J. A., Gulley, M. L., Pathmanathan, R. & Raab-Traub, N. Differential signaling pathways are activated in the Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res. 64, 5251–5260 (2004).
Nagel, S. et al. HLXB9 activates IL6 in Hodgkin lymphoma cell lines and is regulated by PI3K signalling involving E2F3. Leukemia 19, 841–846 (2005).
Renné, C. et al. High expression of several tyrosine kinases and activation of the PI3K/AKT pathway in mediastinal large B cell lymphoma reveals further similarities to Hodgkin lymphoma. Leukemia 21, 780–787 (2007).
Dutton, A., Reynolds, G. M., Dawson, C. W., Young, L. S. & Murray, P. G. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J. Pathol. 205, 498–506 (2005).
Gough, N. R. Focus Issue: demystifying mTOR signaling. Sci. Signal. 2, eg5 (2009).
Dancey, J. mTOR signaling and drug development in cancer. Nat. Rev. Clin. Oncol. 7, 209–219 (2010).
Thomson, A. W., Turnquist, H. R. & Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9, 324–337 (2009).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Younes, A. Therapeutic activity of mTOR inhibitors in mantle cell lymphoma: clues but no clear answers. Autophagy 4, 707–709 (2008).
Zheng, Y. et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol. 178, 2163–2170 (2007).
Del Bufalo, D. et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 66, 5549–5554 (2006).
Younes, A. Therapeutic activity of mTOR inhibitors in mantle cell lymphoma: clues but no clear answers. Autophagy 4, 707–709 (2008).
Smith, S. M. et al. Activity of single agent temsirolimus (CCI-779) in non-mantle cell non-Hodgkin lymphoma subtypes. J. Clin. Oncol. ASCO Meeting Abstracts 26 (Suppl.), 8514 (2008).
Witzig, T. E. et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol. 23, 5347–5356 (2005).
Hess, G. et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 27, 3822–3829 (2009).
Courtney, K. D., Corcoran, R. B. & Engelman, J. A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 28, 1075–1083 (2010).
Zhao, L. & Vogt, P. K. Class I PI3K in oncogenic cellular transformation. Oncogene 27, 5486–5496 (2008).
Lannutti, B. J. et al. CAL-101, an oral p110{delta} selective phosphatidylinositol-3-kinase (PI3K) inhibitor for the treatment of B cell malignancies inhibits PI3K signaling, cellular viability and protective signals of the microenvironment. Blood (ASH Annual Meeting Abstracts) 114, 286 (2009).
Flinn, I. W. et al. evidence of clinical activity in a phase 1 study of CAL-101, an oral P110{delta} isoform-selective inhibitor of phosphatidylinositol 3-kinase, in patients with relapsed or refractory B-cell malignancies. Blood (ASH Annual Meeting Abstracts) 114, 922 (2009).
Fisher, R. I. et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 24, 4867–4874 (2006).
Blum, K. A. et al. Single agent bortezomib in the treatment of relapsed and refractory Hodgkin lymphoma: cancer and leukemia Group B protocol 50206. Leuk. Lymphoma 48, 1313–1319 (2007).
Younes, A., Pro, B. & Fayad, L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood 107, 1731–1732 (2006).
Dunleavy, K. et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood 113, 6069–6076 (2009).
Xu, W. S., Parmigiani, R. B. & Marks, P. A. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26, 5541–5552 (2007).
Duvic, M. et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109, 31–39 (2007).
Piekarz, R. L. et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 27, 5410–5417 (2009).
Piekarz, R. et al. Final results of a phase 2 NCI multicenter study of romidepsin in patients with relapsed peripheral T-cell lymphoma (PTCL). Blood (ASH Annual Meeting Abstracts) 114, 1657 (2009).
Younes, A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am. Soc. Hematol. Educ. Program 507–519 (2009).
Younes, A. et al. Isotype-selective HDAC inhibitor MGCD0103 decreases serum TARC concentrations and produces clinical responses in heavily pretreated patients with relapsed classical Hodgkin lymphoma (HL). Blood (ASH Annual Meeting Abstracts) 110, 2566 (2007).
Kirschbaum, M. H. et al. Vorinostat (suberoylanilide hydroxamic acid) in relapsed or refractory Hodgkin lymphoma: SWOG 0517. Blood (ASH Annual Meeting Abstracts) 110, 2574 (2007).
Spencer, A. et al. Oral panobinostat (LBH589), a novel deacetylase inhibitor (DACi) demonstrates clinical activity in relapsed/refractory Hodgkin lymphoma (HL) [abstract]. Ann. Oncol. 19, a136 (2008).
Younes, A. et al. Efficacy of panobinostat in phase II study in patients with relapsed/refractory Hodgkin lymphoma (HL) after high-dose chemotherapy with autologous stem cell transplant. Blood (ASH Annual Meeting Abstracts) 114, 923 (2009).
Witzig, T. E. et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's lymphoma. J. Clin. Oncol. 27, 5404–5409 (2009).
Witzig, T. E. et al. Durable responses after lenalidomide oral monotherapy in patients with relapsed or refractory (R/R) aggressive non-Hodgkin's lymphoma (a-NHL): results from an international phase 2 study (CC-5013-NHL-003). Blood (ASH Annual Meeting Abstracts) 114, 1676 (2009).
Fehniger, T. A. et al. A phase II multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 3693 (2009).
Kuruvilla, J. et al. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood (ASH Annual Meeting Abstracts) 112, 3052 (2008).
Fowler, N. et al. A biologic combination of lenalidomide and rituximab for front-line therapy of indolent B-cell non-Hodgkin's lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 1714 (2009).
Wang, L. et al. A phase I/II study of lenalidomide in combination with rituximab in relapsed/refractory mantle cell lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 2719 (2009).
Chen, L. et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111, 2230–2237 (2008).
Friedberg, J. W. et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2010).
Pollyea, D. A. et al. A phase I dose escalation study of the Btk inhibitor PCI-32765 in relapsed and refractory B cell non-Hodgkin lymphoma and use of a novel fluorescent probe pharmacodynamic assay. Blood (ASH Annual Meeting Abstracts) 114, 3713 (2009).
Cheson, B. D. et al. Safety and efficacy of YM155 in diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. ASCO Meeting Abstracts 27 (Suppl.), 8502 (2009).
Tolcher, A. W. et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J. Clin. Oncol. 26, 5198–5203 (2008).
Wilson, W. et al. ABT-263 activity and safety in patients with relapsed or refractory lymphoid malignancies in particular chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). J. Clin. Oncol. ASCO Meeting Abstracts 27 (Suppl.), 8574 (2009).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Wiezorek, J., Holland, P. & Graves, J. Death receptor agonists as a targeted therapy for cancer. Clin. Cancer Res. 16, 1701–1708 (2010).
Wu, D. et al. No evidence for the JAK2 (V617F) or JAK2 exon 12 mutations in primary mediastinal large B-cell lymphoma. Diagn. Mol. Pathol. 18, 144–149 (2009).
Melzner, I., Weniger, M. A., Menz, C. K. & Möller, P. Absence of the JAK2 V617F activating mutation in classical Hodgkin lymphoma and primary mediastinal B-cell lymphoma. Leukemia 20, 157–158 (2006).
Weniger, M. A. et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene 25, 2679–2684 (2006).
Melzner, I. et al. Biallelic deletion within 16p13.13 including SOCS-1 in Karpas1106P mediastinal B-cell lymphoma line is associated with delayed degradation of JAK2 protein. Int. J. Cancer 118, 1941–1944 (2006).
Joliot, V., Cormier, F., Medyouf, H., Alcalde, H. & Ghysdael, J. Constitutive STAT5 activation specifically cooperates with the loss of p53 function in B-cell lymphomagenesis. Oncogene 25, 4573–4584 (2006).
Nagy, Z. S. et al. STAT5 regulation of BCL10 parallels constitutive NFkappaB activation in lymphoid tumor cells. Mol. Cancer 8, 67 (2009).
Scheeren, F. A. et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood 111, 4706–4715 (2008).
Kube, D. et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood 98, 762–770 (2001).
Alas, S. & Bonavida, B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin. Cancer Res. 9, 316–326 (2003).
Amin, H. M. et al. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene 23, 5426–5434 (2004).
Holtick, U. et al. STAT3 is essential for Hodgkin lymphoma cell proliferation and is a target of tyrphostin AG17 which confers sensitization for apoptosis. Leukemia 19, 936–944 (2005).
Yared, M. A., Khoury, J. D., Medeiros, L. J., Rassidakis, G. Z. & Lai, R. Activation status of the JAK/STAT3 pathway in mantle cell lymphoma. Arch. Pathol. Lab. Med. 129, 990–996 (2005).
Ding, B. B. et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 111, 1515–1523 (2008).
Younes, A. et al. Phase-I study of the novel oral JAK-2 inhibitor SB1518 in patients with relapsed lymphoma: evidence of clinical and biologic activity. Blood (ASH Annual Meeting Abstracts) 114, 588 (2009).
LoRusso, P. M., Boerner, S. A. & Seymour, L. An overview of the optimal planning, design, and conduct of phase I studies of new therapeutics. Clin. Cancer Res. 16, 1710–1718 (2010).
Hunsberger, S., Rubinstein, L. V., Dancey, J. & Korn, E. L. Dose escalation trial designs based on a molecularly targeted endpoint. Stat. Med. 24, 2171–2181 (2005).
Korn, E. L. Nontoxicity endpoints in phase I trial designs for targeted, non-cytotoxic agents. J. Natl Cancer Inst. 96, 977–978 (2004).
Barker, A. D. et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 86, 97–100 (2009).
Younes, A. et al. Safety and tolerability of conatumumab in combination with bortezomib or vorinostat in patients with relapsed or refractory lymphoma. Blood (ASH Annual Meeting Abstracts) 114, 1708 (2009).
Satoh, T. et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin. Cancer Res. 15, 3872–3880 (2009).
Linardou, H., Dahabreh, I. J., Bafaloukos, D. & Murray, S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat. Rev. Clin. Oncol. 6, 352–366 (2000).
Dhomen, N. & Marais, R. BRAF signaling and targeted therapies in melanoma. Hematol. Oncol. Clin. North Am. 23, 529–545 (2009).
Lieberman, R. Personalized medicine enters the US marketplace: KRAS, anti-EGFR monoclonal antibodies, and colon cancer. Am. J. Ther. 16, 477–479 (2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Cheson, B. D. et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 25, 579–586 (2007).
Flaherty, K. et al. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J. Clin. Oncol. ASCO Meeting Abstracts 27 (Suppl.), 9000 (2009).
Coiffier, B. et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 92, 1927–1932 (1998).
Leonard, J. P. et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin. Cancer Res. 10, 5327–5334 (2004).
O'Mahony, D. et al. Yttrium-90 radiolabeled humanized monoclonal antibody to CD25 in refractory and relapsed Hodgkin's lymphoma. Blood (ASH Annual Meeting Abstracts) 112, 231 (2008).
Viviani, S., Bonfante, V., Fasola, C., Valagussa, P. & Gianni, A. M. Phase II study of the histone-deacetylase inhibitor ITF2357 in relapsed/refractory Hodgkin's lymphoma patients. J. Clin. Oncol. ASCO Meeting Abstracts 26 (Suppl.), 8532 (2008).
Fehniger, T. A. et al. A phase II multicenter study of lenalidomide in patients with relapsed or refractory classical Hodgkin lymphoma (cHL): preliminary results. Blood (ASH Annual Meeting Abstracts) 112, 2595 (2008).
Johnston, P. B. et al. mTOR inhibition for relapsed or refractory Hodgkin lymphoma: promising single agent activity with everolimus (RAD001). Blood (ASH Annual Meeting Abstracts) 110, 2555 (2007).
Reeder, C. B. et al. A phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed aggressive non-Hodgkin lymphoma (NHL). Blood (ASH Annual Meeting Abstracts) 110, 121 (2007).
Ansell, S. M. et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer 113, 508–514 (2008).
Goy, A. et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 23, 667–675 (2005).
Crump, M. et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann. Oncol. 19, 964–969 (2008).
Younes, A. et al. Treatment of relapsed or refractory lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: interim results from a phase II study. Blood (ASH Annual Meeting Abstracts) 110, 2571 (2007).
Robertson, M. J. et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 25, 1741–1746 (2007).
Acknowledgements
This work is partially supported by lymphoma SPORE grant 1P50CA136411-01A1, Clay Chiles Lymphoma Fund, Jack L. Stotsky Memorial Fund, and the Living Legend Fund.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary Figure 1
Rationale for targeting histone deacetylases (HDACs) in cancer. (DOC 99 kb)
Rights and permissions
About this article
Cite this article
Younes, A. Beyond chemotherapy: new agents for targeted treatment of lymphoma. Nat Rev Clin Oncol 8, 85–96 (2011). https://doi.org/10.1038/nrclinonc.2010.189
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.189
This article is cited by
-
Protease nexin-1 prevents growth of human B cell lymphoma via inhibition of sonic hedgehog signaling
Blood Cancer Journal (2018)
-
The landscape of new drugs in lymphoma
Nature Reviews Clinical Oncology (2017)
-
The novel PI3K-δ inhibitor TGR-1202 enhances Brentuximab Vedotin-induced Hodgkin lymphoma cell death via mitotic arrest
Leukemia (2016)
-
Dual PI3K/ERK inhibition induces necroptotic cell death of Hodgkin Lymphoma cells through IER3 downregulation
Scientific Reports (2016)
-
Post-autologous transplant maintenance therapies in lymphoid malignancies: are we there yet?
Bone Marrow Transplantation (2015)