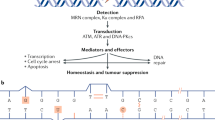
Key Points
-
The metabolism of xenobiotics by xenobiotic-metabolizing enzymes (XMEs) has been classified into phase I (functionalization) and phase II (conjugation) reactions. Cytochrome P450 (CYP) enzymes comprise 70–80% of all phase I XMEs.
-
Although originally thought to be responsible for drug metabolism almost exclusively in the liver, it has now been realized that all XMEs participate in many crucial endogenous functions, probably in every eukaryotic cell and many prokaryotes.
-
Of the 57 human CYP genes in 18 families, the members of the CYP1 to CYP4 families oxygenate thousands of xenobiotics (and endogenous substrates), whereas all members of the CYP5 family and higher principally metabolize endogenous substrates in a highly substrate-specific manner.
-
CYP enzymes can cause tumour initiation through the formation of reactive intermediates (from exogenous and endogenous substrates). CYPs also participate in tumour initiation and progression through inflammation, other eicosanoid-mediated processes and other signal-transduction pathways.
-
There is an interesting 'xenosensor' relationship that is not yet well understood among CYPs, XME receptors that regulate CYP expression and xenobiotic-related transporters (XRTs).
-
Although several high-penetrance, predominantly monogenic (hPpM), traits including cancer have been correlated with various human CYP genes, the identification of a specific procarcinogen is rarely possible.
-
Pharmacokinetic studies of several environmental toxicants in Cyp1 knockout mouse lines have shown that CYP1A1, CYP1A2 and CYP1B1 might be beneficial or detrimental — depending on their time-specific, organ-specific, tissue-specific and cell-type-specific expression. These new findings suggest that animal studies and human epidemiological studies might need to be revisited.
-
Whereas oral drugs more commonly use the portal vein system (and first-pass elimination kinetics), we suggest that the lymphatic system might be more important in delivering the very hydrophobic oral polycyclic aromatic hydrocarbons (PAHs) and polyhalogenated aromatic hydrocarbons (PHAHs) to target tissues. This is especially relevant to clinical medicine, because oral exposure to these procarcinogens is the most predominant route. More focus on pharmacokinetic and pharmacodynamic studies of hydrophobic procarcinogens in animal models (and extrapolation to humans) is needed.
-
A two-tiered model is proposed for the risk of developing cancer as a result of the environment. First, human inter-individual differences in the up-front hPpM traits, encoded by phase I genes, should have profound effects on a small (5–15%) proportion of any population who have no significant polymorphisms in downstream target genes. Second, all downstream targets of phase-I-mediated reactive intermediates can have their own important polymorphisms, always resulting in a unimodal gradient response.
Abstract
Some cytochrome P450 (CYP) heme-thiolate enzymes participate in the detoxication and, paradoxically, the formation of reactive intermediates of thousands of chemicals that can damage DNA, as well as lipids and proteins. CYP expression can also affect the production of molecules derived from arachidonic acid, and alters various downstream signal-transduction pathways. Such changes can be precursors to malignancy. Recent studies in mice have changed our perceptions about the function of CYP1 enzymes. We suggest a two-tiered system to predict an overall inter-individual risk of tumorigenesis based on DNA variants in certain 'early defence' CYP genes, combined with polymorphisms in various downstream target genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nebert, D. W. & Russell, D. W. Clinical importance of the cytochromes P450. Lancet 360, 1155–1162 (2002). One of only a handful of CYP reviews that emphasizes the clinical relevance of CYP enzymes, instead of CYP metabolism of drugs or environmental procarcinogens.
Nelson, D. R. et al. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14, 1–18 (2004). A comparison of all CYP genes in the human genome with all CYP genes in the mouse genome by BLAST searches with each and every CYP exon, resulting in the discovery of many pseudogenes, partial pseudogenes and detritus exons.
Nelson, D. R. et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6, 1–42 (1996).
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 (2006).
Fortini, P. et al. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat. Res. 531, 127–139 (2003).
Undevia, S. D., Gomez-Abuin, G. & Ratain, M. J. Pharmacokinetic variability of anticancer agents. Nature Rev. Cancer 5, 447–458 (2005).
Williams, R. T. Detoxication Mechanisms: The Metabolism of Drugs and Allied Organic Compounds (Chapman and Hall, London, 1949).
Evans, W. E. & Relling, M. V. Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286, 487–491 (1999).
Williams, R. T. The metabolism of certain drugs and food chemicals in man. Ann. N. Y. Acad. Sci. 179, 141–154 (1971). The classical field of drug metabolism is reviewed for the last time by one of the most famous scientists in that field, Richard Tecwyn Williams. He died in 1979 at the age of 70.
Nebert, D. W., Jorge-Nebert, L. & Vesell, E. S. Pharmacogenomics and 'individualized drug therapy': high expectations and disappointing achievements. Am. J. Pharmacogenomics 3, 361–370 (2003).
Ambrosone, C. B. et al. Cigarette smoking, N-acetyltransferase-2 genetic polymorphisms, and breast cancer risk. JAMA 276, 1494–1501 (1996).
Hishida, A. et al. GSTT1 and GSTM1 deletions, NQO1 C609T polymorphism and risk of chronic myelogenous leukemia in Japanese. Asian Pac. J. Cancer Prev. 6, 251–255 (2005).
Landi, S. et al. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet. Genomics 15, 535–546 (2005).
Nebert, D. W. Polymorphisms in drug-metabolizing enzymes: what is their clinical relevance and why do they exist? Am. J. Hum. Genet. 60, 265–271 (1997).
Omura, T. & Sato, R. A new cytochrome in liver microsomes. J. Biol. Chem. 237, 1375–1376 (1962).
Bachschmid, M., Schildknecht, S. & Ullrich, V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun. 338, 536–542 (2005).
Nebert, D. W. et al. The P450 gene superfamily: recommended nomenclature. DNA 6, 1–11 (1987).
Nebert, D. W. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differen-tiation, and neuroendocrine functions. Mol. Endocrinol. 5, 1203–1214 (1991). The review that first predicted the regulatory loops and interactive exogenous and endogenous signalling pathways involving the up-regulation of XMEs by their XME receptors.
Nebert, D. W. & Dieter, M. Z. The evolution of drug metabolism. Pharmacology 61, 124–135 (2000).
Ingelman-Sundberg, M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol. Sci. 25, 193–200 (2004).
Nebert, D. W. & Vesell, E. S. Advances in pharmacogenomics and individualized drug therapy: exciting challenges that lie ahead. Eur. J. Pharmacol. 500, 267–280 (2004). Contrary to more than 1,000 reviews that predict that 'pharmacogenomics will be successful in individualizing drug therapy very soon', this review points out the many difficulties with trying to determine an unequivocal phenotype or genotype.
Johansson, I. et al. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultra-rapid metabolism of debrisoquine. Proc. Natl Acad. Sci. USA 90, 11825–11829 (1993).
Wattenberg, L. W. Effects of dietary constituents on the metabolism of chemical carcinogens. Cancer Res. 35, 3326–3331 (1975).
Conney, A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 42, 4875–4917 (1982). This review summarizes the cutting-edge research of 'enzyme induction' by Allan H. Conney and colleagues that spans the previous three decades.
Cerutti, P. A. Prooxidant states and tumor promotion. Science 227, 375–381 (1985).
Guengerich, F. P. Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res. 48, 2946–2954 (1988). Describes the research of the Guengerich laboratory and other laboratories in elucidating the relationship between CYP enzyme activities and both tumorigenesis and cancer chemotherapy – spanning the previous two decades.
Nebert, D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit. Rev. Toxicol. 20, 153–174 (1989).
Kouri, R. E. et al. Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryopreserved lymphocytes. Cancer Res. 42, 5030–5037 (1982).
Kouri, R. E., McLemore, T., Jaiswal, A. K. & Nebert, D. W. Current cellular assays for measuring clinical drug metabolizing capacity––impact of new molecular biologic techniques. Progr. Clin. Biol. Res. 214, 453–469 (1986).
Idle, J. R. et al. Some observations on the oxidation phenotype status of Nigerian patients presenting with cancer. Cancer Lett. 11, 331–338 (1981).
Ayesh, R., Idle, J. R., Ritchie, J. C., Crothers, M. J. & Hetzel, M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature 312, 169–170 (1984).
Mahgoub, A., Idle, J. R., Dring, L. G., Lancaster, R. & Smith, R. L. Polymorphic hydroxylation of debrisoquine in man. Lancet 2, 584–586 (1977).
Caporaso, N. et al. Lung cancer risk, occupational exposure, and the debrisoquine metabolic phenotype. Cancer Res. 49, 3675–3679 (1989).
Mossman, B. T., Lounsbury, K. M. & Reddy, S. P. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am. J. Respir. Cell Mol. Biol. 34, 666–669 (2006).
Wolf, C. R. et al. CYP2D6 genotyping and the association with lung cancer susceptibility. Pharmacogenetics 4, 104–106 (1994).
London, S. J., Daly, A. K., Thomas, D. C., Caporaso, N. E. & Idle, J. R. Methodological issues in the interpretation of studies of the CYP2D6 genotype in relation to lung cancer risk. Pharmacogenetics 4, 107–108 (1994).
Nebert, D. W., Dalton, T. P., Okey, A. B. & Gonzalez, F. J. Role of aryl hydrocarbon receptor-mediated induc-tion of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 279, 23847–23850 (2004).
Currier, N. et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol. Pathol. 33, 726–737 (2005).
Ohtake, F. et al. Modulation of oestrogen receptor signaling by association with the activated dioxin receptor. Nature 423, 545–550 (2003).
Puga, A. et al. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein poten-tiates repression of E2F-dependent transcription and cell cycle arrest. J. Biol. Chem. 275, 2943–2950 (2000). The most recent paper to review the many functions of the aryl hydrocarbon receptor; not only does the AHR regulate drug-metabolizing and xenobiotic-metabolizing enzymes, but the AHR participates in many signal transduction pathways associated with embryonic and fetal development, central nervous system development and the cell cycle.
Park, K. T., Mitchell, K. A., Huang, G. & Elferink, C. J. The aryl hydrocarbon receptor predisposes hepato-cytes to Fas-mediated apoptosis. Mol. Pharmacol. 67, 612–622 (2005).
Nebert, D. W. et al. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 59, 65–85 (2000).
Cribb, A. E. et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol. Biomarkers Prev. 15, 551–558 (2006).
Sarfarazi, M. & Stoilov, I. Molecular genetics of primary congenital glaucoma. Eye 14 (Pt 3B), 422–428 (2000).
Jiang, Z. et al. Search for an association between the human CYP1A2 genotype and CYP1A2 metabolic phenotype. Pharmacogenet. Genomics 16, 359–367 (2006).
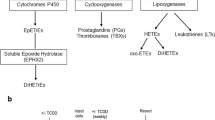
Capdevila, J. H., Harris, R. C. & Falck, J. R. Microsomal cytochrome P450 and eicosanoid metabolism. Cell Mol. Life Sci. 59, 780–789 (2002). Latest review by Jorge Capdevila, who has championed the field showing that many (if not all) CYP enzymes in the CYP1, CYP2, CYP3 and CYP4 gene families participate in both the synthesis and degradation of innumerable eicosanoids.
Nebert, D. W. Drug-metabolizing enzymes in ligand-modulated transcription. Biochem. Pharmacol. 47, 25–37 (1994).
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
Diaz-Cruz, E. S. & Brueggemeier, R. W. Interrelationships between cyclooxygenases and aromatase: unraveling the relevance of cyclooxygenase inhibitors in breast cancer. Anticancer Agents Med. Chem. 6, 221–232 (2006).
Telliez, A., Furman, C., Pommery, N. & Henichart, J. P. Mechanisms leading to COX2 expression and COX2-induced tumorigenesis: topical therapeutic strategies targeting COX2 expression and activity. Anticancer Agents Med. Chem. 6, 187–208 (2006).
Murakami, M. & Kudo, I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr. Pharm. Des 12, 943–954 (2006).
Nebert, D. W. in Toward a Molecular Basis of Alcohol Use and Abuse (eds Jansson, B., Jörnvall, H., Rydberg, U., Terenius, L. & Vallee, B.) 231–240 (Nobel Symposium Press, Stockholm, 1994).
Li, A. G., Lu, S. L., Han, G., Hoot, K. E. & Wang, X. J. Role of TGFβ in skin inflammation and carcinogenesis. Mol. Carcinog. 45, 389–396 (2006).
Clevers, H. At the crossroads of inflammation and cancer. Cell 118, 671–674 (2004).
Mannering, G. J., Shoeman, J. A. & Deloria, L. B. Identification of the antibiotic hops component, colupulone, as an inducer of hepatic cytochrome P-4503A in the mouse. Drug Metab. Dispos. 20, 142–147 (1992).
Qatanani, M. & Moore, D. D. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr. Drug Metab. 6, 329–339 (2005).
Kalaany, N. Y. & Mangelsdorf, D. J. LXRs and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 68, 159–191 (2006).
Zelcer, N. & Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 (2006).
Nebert, D. W. & McKinnon, R. A. Cytochrome P450: evolution and functional diversity. Prog. Liver Dis. 12, 63–97 (1994).
Clowes, J. A., Riggs, B. L. & Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 208, 207–227 (2005).
Chawla, A., Repa, J. J., Evans, R. M. & Mangelsdorf, D. J. Nuclear receptors and lipid physiology: opening the X-files. Science 294, 1866–1870 (2001).
Kakizaki, S., Karami, S. & Negishi, M. Retinoic acids repress constitutive active receptor-mediated induction by 1, 4-bis[2-(3, 5-dichloropyridyloxy)]benzene of the Cyp2b10 gene in mouse primary hepatocytes. Drug Metab. Dispos. 30, 208–211 (2002).
Bikle, D. D., Xie, Z., Ng, D., Tu, C. L. & Oda, Y. Squamous cell carcinomas fail to respond to the prodifferentiating actions of 1, 25-(OH)2-vitamin D: why? Recent Results Cancer Res. 164, 111–122 (2003).
Chen, G. & White, P. A. The mutagenic hazards of aquatic sediments: a review. Mutat. Res. 567, 151–225 (2004).
Jansen, M. S. et al. Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 101, 7199–7204 (2004).
Geier, A. et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 38, 345–354 (2003).
Grover, P. L. & Sims, P. Enzyme-catalysed reactions of polycyclic hydrocarbons with deoxyribonucleic acid and protein in vitro. Biochem. J. 110, 159–160 (1968). This 2-page publication was 'the shot heard 'round the world'. Their findings completely changed the thinking of most colleagues in the field for the next three decades.
Dalton, T. P. et al. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem. Biophys. Res. Commun. 267, 184–189 (2000).
Liang, H. C. et al. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc. Natl Acad. Sci. USA 93, 1671–1676 (1996).
Buters, J. T. et al. Cytochrome P450 CYP1B1 deter-mines susceptibility to 7, 12-dimethylbenz[a]anth-racene-induced lymphomas. Proc. Natl Acad. Sci. USA 96, 1977–1982 (1999).
Harstad, E. B., Guite, C. A., Thomae, T. L. & Bradfield, C. A. Liver deformation in Ahr-null mice: evidence for aberrant hepatic perfusion in early development. Mol. Pharmacol. 69, 1534–1541 (2006).
Fernandez-Salguero, P. et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268, 722–726 (1995).
Uno, S. et al. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 65, 1225–1237 (2004).
Uno, S. et al. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol. Pharmacol. 69, 1103–1114 (2006).
Tsuneoka, Y. et al. 4-Aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(−/−) and Cyp1a2(+/+) mice. J. Natl Cancer Inst. 95, 1227–1237 (2003).
Ma, X. et al. Mouse lung CYP1A1 catalyzes the metabolic activation of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP). Carcinogenesis 25 Oct 2006 [epub ahead of print].
Mohr, U., Emura, M., Aufderheide, M., Riebe, M. & Ernst, H. Possible role of genetic predisposition in multi-generation carcinogenesis. IARC Sci. Publ. 96, 93–103 (1989).
Dragin, N., Dalton, T. P., Miller, M. L., Shertzer, H. G. & Nebert, D. W. For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. J. Biol. Chem. 281, 18591–18600 (2006). Shows that maternal (mouse or human) hepatic CYP1A2 acts like a 'sink' to sequester dioxin (and presumably other extremely hydrophobic coplanar environmental toxicants), resulting in a substantial decrease in the amount of said environmental toxicant reaching the developing baby in utero.
Eaton, D. L., Gallagher, E. P., Bammler, T. K. & Kunze, K. L. Role of cytochrome P450 1A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics 5, 259–274 (1995).
Nebert, D. W., McKinnon, R. A. & Puga, A. Human drug-metabolizing enzyme polymorphisms: effects on risk of toxicity and cancer. DNA Cell Biol. 15, 273–280 (1996).
Feng, B. Y. & Shoichet, B. K. Synergy and antagonism of promiscuous inhibition in multiple-compound mixtures. J. Med. Chem. 49, 2151–2154 (2006).
Okey, A. B., Boutros, P. C. & Harper, P. A. Polymorphisms of human nuclear receptors that control expression of drug-metabolizing enzymes. Pharmacogenet. Genomics 15, 371–379 (2005).
Cybulski, C. et al. CHEK2 is a multi-organ cancer susceptibility gene. Am. J. Hum. Genet. 75, 1131–1135 (2004).
Stacey, S. N. et al. The BARD1 Cys557Ser variant and breast cancer risk in Iceland. PLoS Med. 3, e217 (2006).
Nelson, D. R. Cytochrome P450 and the individuality of species. Arch. Biochem. Biophys. 369, 1–10 (1999).
Routledge, P. A. & Shand, D. G. Presystemic drug elimination. Annu. Rev. Pharmacol. Toxicol. 19, 447–468 (1979). Describes for the first time the concept and mech-anism of 'first-pass elimination kinetics', involving drug absorption from the gastro-intestinal tract, which can follow the oral administration of drugs.
Nestel, P. J., Havel, R. J. & Bezman, A. Sites of initial removal of chylomicron triglyceride fatty acids from the blood. J. Clin. Invest. 41, 1915–1921 (1962).
Nestel, P. J., Havel, R. J. & Bezman, A. Metabolism of constituent lipids of dog chylomicrons. J. Clin. Invest. 42, 1313–1321 (1963).
Stary, H. C. Macrophages, macrophage foam cells, and eccentric intimal thickening in the coronary arteries of young children. Atherosclerosis 64, 91–108 (1987).
Norum, K. R. & Blomhoff, R. McCollum Award Lecture, 1992: vitamin A absorption, transport, cellular uptake, and storage. Am. J. Clin. Nutr. 56, 735–744 (1992).
Cooper, A. D. Hepatic uptake of chylomicron remnants. J. Lipid Res. 38, 2173–2192 (1997).
Yu, A. M., et al. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics 13, 173–181 (2003). Describes how the insertion of a BAC clone into the mouse genome, to make a humanized CYP2D6 mouse line still carrying all nine of its functional Cyp2d mouse genes, was successful in showing that the human CYP2D6 enzyme participates in serotonin biosynthesis.
Jiang, Z. et al. Toward the evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC-transgenic mice carrying the human CYP1A1_CYP1A2 locus. Hum. Mutat. 25, 196–206 (2005). Among the first publications to detect all double-hit and single-hit SNPs across the 39.6 kb of the human CYP1A1–CYP1A2 locus, delineation of SNPs, and SNP allelic frequencies in African, East Asian and Caucasian populations. Also describes the generation of a humanized CYP1A1–CYP1A2 mouse line.
Cheung, C. et al. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4, 5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem. Res. Toxicol. 18, 1471–1478 (2005).
Derkenne, S. et al. Theophylline pharmacokinetics: comparison of Cyp1a1(−/−) and Cyp1a2(−/−) knockout mice, humanized hCYP1A1_1A2 knock-in mice lacking either the mouse Cyp1a1 or Cyp1a2 gene, and Cyp1(+/+) wild-type mice. Pharmacogenet. Genomics 15, 503–511 (2005).
Moriguchi, T. et al. Distinct response to dioxin in an aryl hydrocarbon receptor (AHR)-humanized mouse. Proc. Natl Acad. Sci. USA 100, 5652–5657 (2003).
Court DL, Sawitzke, J. A. & Thomason, L. C. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36, 361–388 (2002)
Conney, A. H., Chang, R. L., Jerina, D. M. & Wei, S. J. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 26, 125–163 (1994).
DeCaprio, A. P. The toxicology of hydroquinone — relevance to occupational and environmental exposure. Crit. Rev. Toxicol. 29, 283–330 (1999).
Nemoto, N., Hirakawa, T. & Takayama, S. Effect of UDP glucuronic acid on the microsome-mediated binding of benzo[a]pyrene metabolites to calf thymus DNA. Carcinogenesis 1, 115–127 (1980).
Wollemann, M. & Feuer, G. Formation of fluoroacetyl-coenzyme A and fluoroacetylcholine from fluoroacetic acid and fluorocitric acid in brain extracts. Acta Physiol. Hung. 11, 165–172 (1957).
Monks, T. J. et al. Glutathione conjugate-mediated toxicities. Toxicol. Appl. Pharmacol. 106, 1–19 (1990).
Gunaratnam, M. & Grant, M. H. The role of glutathione reductase in the cytotoxicity of chromium (VI) in isolated rat hepatocytes. Chem. Biol. Interact. 134, 191–202 (2001).
Pourahmad, J. & O'Brien, P. J. Biological reactive intermediates that mediate chromium (VI) toxicity. Adv. Exp. Med. Biol. 500, 203–207 (2001).
Gunaratnam, M., Pohlscheidt, M. & Grant, M. H. Pretreatment of rats with the inducing agents phenobarbitone and 3-methylcholanthrene ameliorates the toxicity of chromium (VI) in hepatocytes. Toxicol. In Vitro 16, 509–516 (2002).
Porter, R., Jachymova, M., Martasek, P., Kalyanaraman, B. & Vasquez-Vivar, J. Reductive activation of Cr(Vi) by nitric oxide synthase. Chem. Res. Toxicol. 18, 834–843 (2005).
Nemeti, B. & Gregus, Z. Glutathione-dependent reduction of arsenate in human erythrocytes — a process independent of purine nucleoside phosphorylase. Toxicol. Sci. 82, 419–428 (2004).
Nemeti, B. & Gregus, Z. Reduction of arsenate to arsenite by human erythrocyte lysate and rat liver cytosol: characterization of a glutathione- and NAD-dependent arsenate reduction linked to glycolysis. Toxicol. Sci. 85, 847–858 (2005).
Nemeti, B., Csanaky, I. & Gregus, Z. Effect of an inactivator of glyceraldehyde-3-phosphate dehydrogenase, a fortuitous arsenate reductase, on disposition of arsenate in rats. Toxicol. Sci. 90, 49–60 (2006).
Mukherjee, B. et al. Glutathione S-transferase ω-1 and ω-2 pharmacogenomics. Drug Metab. Dispos. 34, 1237–1246 (2006).
Ramakrishna, G. & Anderson, L. M. Levels and membrane localization of the c-K-ras p21 protein in lungs of mice of different genetic strains and effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) and Aroclor 1254. Carcinogenesis 19, 463–470 (1998).
Goth, L., Rass, P. & Pay, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 8, 141–149 (2004).
Cocco, P. Does G6PD deficiency protect against cancer? A critical review. J. Epidemiol. Community Health 41, 89–93 (1987).
Hein, D. W. N-acetyltransferase-2 genetic poly-morphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene 25, 1649–1658 (2006).
Terry, P. D. & Goodman, M. Is the association between cigarette smoking and breast cancer modified by genotype? A review of epidemiologic studies and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15, 602–611 (2006).
Agundez, J. A. Cytochrome P450 gene polymorphism and cancer. Curr. Drug Metab. 5, 211–224 (2004).
Rodriguez-Antona, C. & Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 25, 1679–1691 (2006).
Costa, L. G., Vitalone, A., Cole, T. B. & Furlong, C. E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 69, 541–550 (2005).
Schroeder, J. C. Metabolic susceptibility to agricultural pesticides and non-Hodgkin lymphoma. Scand. J. Work Environ. Health 31 (Suppl. 1), 26–32 (2005).
Crabb, D. W., Matsumoto, M., Chang, D. & You, M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. 63, 49–63 (2004).
Nowell, S. & Falany, C. N. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 25, 1673–1678 (2006).
Nioi, P. & Hayes, J. D. Contribution of NAD(P)H:quinone oxidoreductase-1 to protection against carcinogenesis, and regulation of its gene by the NRF2 basic-region leucine zipper and the aryl hydrocarbon receptor basic helix-loop-helix transcription factors. Mutat. Res. 555, 149–171 (2004).
Kiyohara, C., Yoshimasu, K., Takayama, K. & Nakanishi, Y. EPHX1 polymorphisms and the risk of lung cancer: a HuGE review. Epidemiology 17, 89–99 (2006).
Ye, Z. & Song, H. Glutathione S-transferase polymorphisms (GSTM1, GSTP1 and GSTT1) and the risk of acute leukemia: a systematic review and meta-analysis. Eur. J. Cancer 41, 980–989 (2005).
Lai, R., Crevier, L. & Thabane, L. Genetic polymorphisms of glutathione S-transferases and the risk of adult brain tumors: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 14, 1784–1790 (2005).
Maruo, Y., Iwai, M., Mori, A., Sato, H. & Takeuchi, Y. Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr. Drug Metab. 6, 91–99 (2005).
Plastaras, J. P., Guengerich, F. P., Nebert, D. W. & Marnett, L. J. Xenobiotic-metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J. Biol. Chem. 275, 11784–11790 (2000).
Spiecker, M. & Liao, J. K. Vascular protective effects of cytochrome P450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 433, 413–420 (2005).
Bylund, J. & Oliw, E. H. Cloning and characterization of CYP4F21: a prostaglandin E2 20-hydroxylase of ram seminal vesicles. Arch. Biochem. Biophys. 389, 123–129 (2001).
Parmentier, J. H., Lavrentyev, E. N., Falck, J. R., Capdevila, J. H. & Malik, K. U. Evaluation of cytochrome P450 4 family as mediator of phospholipase D activation in aortic vascular smooth muscle cells. Life Sci. 77, 1015–1029 (2005).
Nebert, D. W. Inter-individual susceptibility to environmental toxicants — a current assessment. Toxicol. Appl. Pharmacol. 207, 34–42 (2005).
Xu, C., Li, C. Y. & Kong, A. N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28, 249–268 (2005).
Tirona, R. G. & Kim, R. B. Nuclear receptors and drug disposition gene regulation. J. Pharm. Sci. 94, 1169–1186 (2005).
Klaassen, C. D. & Slitt, A. L. Regulation of hepatic transporters by xenobiotic receptors. Curr. Drug Metab. 6, 309–328 (2005).
Bookout, A. L. et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 (2006). One of the latest cutting-edge publications to describe receptors and their regulatory functions. A survey of the expression of all 49 mouse NR mRNAs in 39 tissues shows a hierarchical transcriptional circuitry that extends beyond individual tissues to form a mega-network that governs physiology on an organismal scale.
Jandacek, R. J., Zheng, S., Yang, Q. & Tso, P. Rapid clearance of hexachlorobenzene from chylomicrons. Lipids 39, 993–995 (2004).
Acknowledgements
We thank our colleagues, especially L.F. Jorge-Nebert, for valuable discussions and careful readings of this manuscript. This work was funded in part by US National Institutes of Health grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Human Cytochrome P450 Allele Nomenclature Committee
Glossary
- Xenobiotic
-
A foreign chemical in the body. Any chemical, including drugs and foodstuffs, normally located outside the living organism, which exists in the organism (the opposite of an endogenous compound).
- Eicosanoid
-
Any of the many physiologically active substances derived from arachidonic acid — including the prostaglandins, leuko-trienes, prostacyclins and thromboxanes — that are involved in many crucial life functions.
- Inborn errors of metabolism
-
The term coined by A. Garrod in 1908 applying to heritable disorders of biochemistry. Examples include albinism and phenylketonuria.
- Hydrophilic
-
To have a strong affinity for water, which is a property of polar molecules.
- Reactive oxygenated intermediates
-
Chemicals, into which an atom of oxygen has been inserted. ROMs are relatively unstable and degrade quickly by reacting with, and binding covalently to, nucleic acids and proteins.
- Genotoxicity
-
DNA damage. Many reactive oxygenated intermediates attack DNA, and either covalently bind to it to form adducts or produce oxidative stress, which can cause strand breaks and other chromosomal damage.
- Xenobiotic-metabolizing enzymes
-
Enzymes that can metabolize a foreign chemical. It has been suggested that no XME exists for the metabolism of xenobiotics alone, that is, all XMEs have one or more endogenous substrates or functions. Often regulated by 'xenobiotic-sensing' receptors.
- Xenobiotic-related transporters
-
Membrane-bound transporters that move endobiotics (and xenobiotics) across membranes, but which are also regulated by 'xenobiotic-sensing' receptors.
- Pharmacokinetics
-
What the body does to a drug (or other exogenous chemical), encompassing absorption, distribution, metabolism and excretion. Sometimes called 'toxicokinetics'.
- Pharmacodynamics
-
What a drug (or other exogenous chemical) does to the body, encompassing the biochemical and physiological effects and the mechanisms of drug or chemical action. Sometimes called 'toxicodynamics'.
- Detoxication
-
Whereas various enzymes might be described as 'detoxifying a chemical substrate', R.T. Williams, in the 1940s, chose the term 'detoxication' to describe all detoxifying pathways in intact animals.
- Procarcinogens
-
Chemicals capable of causing cancer, but which first require metabolic activation.
- Electrophile
-
Molecules that are electron-deficient, and that are therefore attracted to other molecules (for example, nucleic acids and proteins) at a position where there is a net negative charge.
- Hydrophobic
-
To have little affinity or to be repelled by water, a property of lipophilic ('fat loving') or nonpolar molecules.
- Monogenic
-
Reflecting the properties of a single gene.
- Penetrance
-
The penetrance of a gene refers to the proportion of individuals who, having a defined genotype, manifest a particular trait.
- Multiplex phenotype
-
A trait caused by two or more genes and environmental factors.
- Polycyclic aromatic hydrocarbons
-
Chemicals that contain only C and H atoms arranged in three or more rings (cycles), and that have conjugating double bonds (φ electrons), that is, not saturated. Benzo[α]pyrene (BaP) is a prototypic example. Found in industrial incineration products, cigarette smoke and charcoal-grilled food.
- Aflatoxin B1
-
A potent toxin (C17H12O6) produced by the Aspergillus flavus group of fungi, which causes liver toxicity and cancer in mammals, birds and fish; it also causes mutations, birth defects and immunosuppression in animals. Found as a contaminant in peanuts, cottonseed meal, corn and other grains.
- Polyhalogenated aromatic hydrocarbons
-
Chemicals comprised of C, H, and Cl, Br or F, and sometimes O atoms that are found in toxic waste dump sites and contaminated water; originating from insulators, combustion, refrigeration and other industrial processes. Polychlorinated biphenyls (PCBs) are a prototypic class of PHAHs. PHAHs can be either coplanar or non-coplanar.
- Colupulone
-
The chemical in hops that gives beer its antibiotic properties.
- Microsomes
-
An endoplasmic-reticulum-enriched preparation, produced by separating out cellular subfractions in an ultracentrifuge.
- Ductus venosus
-
The blood vessel, located within the fetal liver, that connects (shunts) the umbilical vein to the inferior vena cava; at the time of birth, changes in the amount of oxygen in the blood and arterial–venous pressure normally close this shunt.
- Methemoglobinemia
-
A condition in which the iron in the haemoglobin molecule (the red blood pigment) is oxidized, making it unable to carry oxygen effectively to the tissues.
- Endobiotic
-
An endogenous compound. Any molecule that is normally expected to be located inside the organism (the opposite of xenobiotic).
- Recombineering
-
The method by which highly efficient bacteriophage-based Escherichia coli homologous recombination sequences (as short as 35–50 bp) can enable genomic DNA in bacterial artificial chromosomes (BACs) to be modified and subcloned, without the need for restriction enzymes or DNA ligases. This new form of chromosome engineering is efficient and significantly reduces the time it takes to create transgenic mouse models compared with traditional methods.
Rights and permissions
About this article
Cite this article
Nebert, D., Dalton, T. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6, 947–960 (2006). https://doi.org/10.1038/nrc2015
Issue Date:
DOI: https://doi.org/10.1038/nrc2015