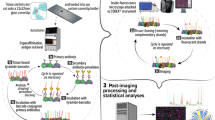
Abstract
Active enzymes, such as proteases, often serve as valuable biomarkers for various disease pathologies. Therefore, methods to detect specific enzyme activities in biological samples can provide information to guide disease detection and diagnosis and to increase our understanding of the biological roles of specific enzyme targets. In this protocol, we outline methods for the topical application of fluorescently quenched activity-based probes (qABPs) to fresh-frozen tissue samples. This technique enables rapid imaging of enzyme activity at cellular resolution, and it can be combined with antibody labeling for immunodiagnosis. In this method, fresh-frozen tissue sections are fixed, incubated with the probe and imaged using fluorescence microscopy. This provides an advance over classical immunohistochemistry (IHC) in that it is rapid (4–8 h) and inexpensive, and it provides information on enzyme activity. Furthermore, it can be used with any of the growing number of fluorescent ABPs to provide data for more effective disease monitoring and diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McMahon, J. et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br. J. Oral Maxillofac. Surg. 41, 224–231 (2003).
Ravasz, L.A., Slootweg, P.J., Hordijk, G.J., Smit, F. & van der Tweel, I. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J. Craniomaxillofac. Surg. 19, 314–318 (1991).
Haque, R., Contreras, R., McNicoll, M.P., Eckberg, E.C. & Petitti, D.B. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. 6, 2 (2006).
Nagtegaal, I.D. & Quirke, P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 26, 303–312 (2008).
Singletary, S.E. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am. J. Surg. 184, 383–393 (2002).
Snijder, R.J., Brutel de la Riviere, A., Elbers, H.J. & van den Bosch, J.M. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann. Thorac. Surg. 65, 212–216 (1998).
Kunos, C. et al. Breast conservation surgery achieving ≥2 mm tumor-free margins results in decreased local-regional recurrence rates. Breast J. 12, 28–36 (2006).
Kong, C.S. & Jensen, K.C. Specimen processing. in Practical Breast Pathology 15–23 (Elsevier Saunders, Expert Consult, 2013).
Bydlon, T.M. et al. Performance metrics of an optical spectral imaging system for intraoperative assessment of breast tumor margins. Opt. Express 18, 8058–8076 (2010).
Jacobs, L. Positive margins: the challenge continues for breast surgeons. Ann. Surg. Oncol. 15, 1271–1272 (2008).
Abraham, S.C., Fox, K., Fraker, D., Solin, L. & Reynolds, C. Sampling of grossly benign breast reexcisions: a multidisciplinary approach to assessing adequacy. Am. J. Surg. Pathol. 23, 316–322 (1999).
Nguyen, Q.T. & Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat. Rev. Cancer 13, 653–662 (2013).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
Blum, G. et al. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat. Chem. Biol. 1, 203–209 (2005).
Cutter, J.L. et al. Topical application of activity-based probes for visualization of brain tumor tissue. PLoS ONE 7, e33060 (2012).
Funovics, M., Weissleder, R. & Tung, C.H. Protease sensors for bioimaging. Anal. Bioanal. Chem. 377, 956–963 (2003).
Segal, E. et al. Detection of intestinal cancer by local, topical application of a quenched fluorescence probe for cysteine cathepsins. Chem. Biol. 22, 148–158 (2015).
Verdoes, M. et al. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J. Am. Chem. Soc. 135, 14726–14730 (2013).
Kos, J., Werle, B., Lah, T. & Brunner, N. Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int. J. Biol. Markers 15, 84–89 (2000).
Fonovic, M. & Turk, B. Cysteine cathepsins and extracellular matrix degradation. Biochim. Biophys. Acta 1840, 2560–2570 (2014).
Repnik, U., Stoka, V., Turk, V. & Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 1824, 22–33 (2012).
Zheng, T. et al. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-γ–induced alveolar remodeling and pulmonary emphysema. J. Immunol. 174, 8106–8115 (2005).
Blum, G., von Degenfeld, G., Merchant, M.J., Blau, H.M. & Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 3, 668–677 (2007).
Withana, N.P. et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 72, 1199–1209 (2012).
Sanman, L.E. & Bogyo, M. Activity-based profiling of proteases. Annu. Rev. Biochem. 83, 249–273 (2014).
Lavy, R. et al. A comparative study on two different pathological methods to retrieve lymph nodes following gastrectomy. Int. J. Surg. 12, 725–728 (2014).
Shi, S.R. et al. Evaluation of the value of frozen tissue section used as “gold standard” for immunohistochemistry. Am. J. Clin. Pathol. 129, 358–366 (2008).
Fischer, A.H., Jacobson, K.A., Rose, J. & Zeller, R. Fixation and permeabilization of cells and tissues. CSH Protoc. 2008 pdb.top36 (2008).
Randall, H.W., Bogdanffy, M.S. & Morgan, K.T. Enzyme histochemistry of the rat nasal mucosa embedded in cold glycol methacrylate. Am. J. Anat. 179, 10–17 (1987).
Verdoes, M. et al. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem. Biol. 19, 619–628 (2012).
Greenbaum, D. et al. Chemical approaches for functionally probing the proteome. Mol. Cell. Proteomics 1, 60–68 (2002).
Edgington, L.E. et al. Functional imaging of legumain in cancer using a new quenched activity-based probe. J. Am. Chem. Soc. 135, 174–182 (2013).
Puri, A.W., Broz, P., Shen, A., Monack, D.M. & Bogyo, M. Caspase-1 activity is required to bypass macrophage apoptosis upon Salmonella infection. Nat. Chem. Biol. 8, 745–747 (2012).
Edgington, L.E. et al. An optimized activity-based probe for the study of caspase-6 activation. Chem. Biol. 19, 340–352 (2012).
Edgington, L.E. et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 15, 967–973 (2009).
Ben-Nun, Y. et al. Photodynamic quenched cathepsin activity based probes for cancer detection and macrophage targeted therapy. Theranostics 5, 847–862 (2015).
Berkers, C.R. et al. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat. Methods 2, 357–362 (2005).
Patricelli, M.P., Giang, D.K., Stamp, L.M. & Burbaum, J.J. Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics 1, 1067–1071 (2001).
Liu, Y., Patricelli, M.P. & Cravatt, B.F. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96, 14694–14699 (1999).
Bogyo, M. et al. Applications for chemical probes of proteolytic activity. Curr. Protoc. Protein Sci. 36, 21.17.1–21.17.35 (2004).
Acknowledgements
We thank P. Chu from the Department of Pathology at Stanford University for assistance with processing the histology samples. We thank C.S. Kong and R. West from the Department of Pathology at Stanford University for intellectual input and pathological analysis of tissue samples. This work was funded by US National Institutes of Health grants R01 HL116307 and R01 EB005011 (to M.B.), by the Howard Hughes Medical Institute Medical Research Fellows program (to M.G.), and by the Stanford Medical Scientist Training Program (to M.G.).
Author information
Authors and Affiliations
Contributions
N.P.W. and M.G. designed and performed all experiments and drafted the manuscript. M.V. and L.O.O. designed and synthesized BMV109 and GB111-NH2 used in experiments. E.S. helped establish the tile scans and obtain tissue samples for analysis. M.B. developed and coordinated the project, analyzed the data and prepared and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Withana, N., Garland, M., Verdoes, M. et al. Labeling of active proteases in fresh-frozen tissues by topical application of quenched activity-based probes. Nat Protoc 11, 184–191 (2016). https://doi.org/10.1038/nprot.2016.004
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.004
This article is cited by
-
Cysteine Cathepsins in Breast Cancer: Promising Targets for Fluorescence-Guided Surgery
Molecular Imaging and Biology (2023)
-
Fundamentals and developments in fluorescence-guided cancer surgery
Nature Reviews Clinical Oncology (2022)
-
Fluorescent image-guided surgery in breast cancer by intravenous application of a quenched fluorescence activity-based probe for cysteine cathepsins in a syngeneic mouse model
EJNMMI Research (2020)
-
High-Resolution Confocal Fluorescence Imaging of Serine Hydrolase Activity in Cryosections – Application to Glioma Brain Unveils Activity Hotspots Originating from Tumor-Associated Neutrophils
Biological Procedures Online (2020)
-
Near-infrared fluorophores for biomedical imaging
Nature Biomedical Engineering (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.