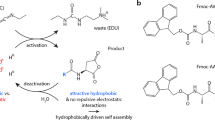
Abstract
This protocol details the preparation of low-molecular-weight hydrogels (LMWGs) in which the gelation time and mechanical stiffness of the final gel can be tuned with the concentration of the catalyst used in the in situ formation of the hydrogelator. By altering the rate of formation of the hydrazone-based gelator from two water-soluble compounds—an oligoethylene functionalized benzaldehyde and a cyclohexane-derived trishydrazide—in the presence of acid or aniline as catalyst, the kinetics of gelation can be tuned from hours to minutes. The resulting materials display controllable stiffness in the 5–50 kPa range. This protocol works at ambient temperatures in water, at either neutral or moderately acidic pH (phosphate buffer, pH 5) depending on the catalyst used. The hydrazide and aldehyde precursors take a total of 5 d to prepare. The final gel is prepared by mixing aqueous solutions of the two precursors and can take between minutes and hours to set, depending on the catalytic conditions. We also describe analysis of the hydrogels by critical gel concentration (CGC) tests, rheology and confocal laser-scanning microscopy (CLSM).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Van Esch, J.H. & Feringa, B.L. New functional materials based on self-assembling organogels: from serendipity towards design. Angew. Chem. Int. Ed. 39, 2263–2266 (2000).
Sangeetha, N.M. & Maitra, U. Supramolecular gels: functions and uses. Chem. Soc. Rev. 34, 821 (2005).
Lee, S.S. et al. Bone regeneration with low-dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 34, 452–459 (2013).
Li, J. et al. Self-delivery multifunctional anti-HIV hydrogels for sustained release. Adv. Healthc. Mater. 2, 1586–1590 (2013).
Adams, D.J., Mullen, L.M., Berta, M., Chen, L. & Frith, W.J. Relationship between molecular structure, gelation behaviour and gel properties of Fmoc-dipeptides. Soft Matter 6, 1971 (2010).
Howe, R.C.T. et al. A family of simple benzene 1,3,5-tricarboxamide (BTA) aromatic carboxylic acid hydrogels. Chem. Commun. 49, 4268 (2013).
Jayawarna, V. et al. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl–dipeptides. Adv. Mater. 18, 611–614 (2006).
Ding, B. et al. Two approaches for the engineering of homogeneous small-molecule hydrogels. Soft Matter 9, 4672 (2013).
Muraoka, T., Cui, H. & Stupp, S.I. Quadruple helix formation of a photoresponsive peptide amphiphile and its light-triggered dissociation into single fibers. J. Am. Chem. Soc. 130, 2946–2947 (2008).
De Jong, J.J.D. et al. Light-driven dynamic pattern formation. Angew. Chem. Int. Ed. 44, 2373–2376 (2005).
Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Weiss, R. & Terech, P. Molecular Gels (Springer, 2006).
Boekhoven, J. et al. Catalytic control over supramolecular gel formation. Nat. Chem. 5, 433–437 (2013).
He, X. et al. Synthetic homeostatic materials with chemo-mechano-chemical self-regulation. Nature 487, 214–218 (2012).
Boekhoven, J., Koot, M., Wezendonk, T.A., Eelkema, R. & van Esch, J.H. A self-assembled delivery platform with post-production tunable release rate. J. Am. Chem. Soc. 134, 12908–12911 (2012).
Montarnal, D., Capelot, M., Tournilhac, F. & Leibler, L. Silica-like malleable materials from permanent organic networks. Science 334, 965–968 (2011).
Williams, R.J. et al. Enzyme-assisted self-assembly under thermodynamic control. Nat. Nanotechnol. 4, 19–24 (2008).
Hirst, A.R. et al. Biocatalytic induction of supramolecular order. Nat. Chem. 2, 1089–1094 (2010).
Zhao, F. et al. β-Galactosidase-instructed formation of molecular nanofibers and a hydrogel. Nanoscale 3, 2859 (2011).
Gao, Y. et al. Enzyme-instructed self-assembly of peptide derivatives to form nanofibers and hydrogels. Biopolymers 94, 19–31 (2010).
Webber, M.J., Newcomb, C.J., Bitton, R. & Stupp, S.I. Switching of self-assembly in a peptide nanostructure with a specific enzyme. Soft Matter 7, 9665 (2011).
Kühnle, H. & Börner, H.G. Biotransformation on polymer-peptide conjugates: a versatile tool to trigger microstructure formation. Angew. Chem. Int. Ed. 48, 6431–6434 (2009).
John, G., Zhu, G., Li, J. & Dordick, J.S. Enzymatically derived sugar-containing self-assembled organogels with nanostructured morphologies. Angew. Chem. Int. Ed. 45, 4772–4775 (2006).
Gao, J. et al. Enzyme promotes the hydrogelation from a hydrophobic small molecule. J. Am. Chem. Soc. 131, 11286–11287 (2009).
Hahn, M.E. & Gianneschi, N.C. Enzyme-directed assembly and manipulation of organic nanomaterials. Chem. Commun. 47, 11814 (2011).
Gao, Y., Shi, J., Yuan, D. & Xu, B. Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nat. Commun. 3, 1033 (2012).
Bachmann, P.A., Luisi, P.L. & Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 357, 57–59 (1992).
Budin, I. & Devaraj, N.K. Membrane assembly driven by a biomimetic coupling reaction. J. Am. Chem. Soc. 134, 751–753 (2012).
Xing, Y., Wang, C., Han, P., Wang, Z. & Zhang, X. Acetylcholinesterase responsive polymeric supra-amphiphiles for controlled self-assembly and disassembly. Langmuir 28, 6032–6036 (2012).
Van Bommel, K.J.C. et al. Responsive cyclohexane-based low-molecular-weight hydrogelators with modular architecture. Angew. Chem. Int. Ed. 43, 1663–1667 (2004).
Dirksen, A., Dirksen, S., Hackeng, T.M. & Dawson, P.E. Nucleophilic catalysis of hydrazone formation and transimination: implications for dynamic covalent chemistry. J. Am. Chem. Soc. 128, 15602–15603 (2006).
Bhat, V.T. et al. Nucleophilic catalysis of acylhydrazone equilibration for protein-directed dynamic covalent chemistry. Nat. Chem. 2, 490–497 (2010).
Ramström, O., Lohmann, S., Bunyapaiboonsri, T. & Lehn, J.-M. Dynamic combinatorial carbohydrate libraries: probing the binding site of the concanavalin a lectin. Chem. Eur. J. 10, 1711–1715 (2004).
Boudjouk, P., Kapfer, C.A. & Cunico, R.F. Synthesis and reactivity of 1-silaadamantyl systems. Organometallics 2, 336–343 (1983).
Acknowledgements
We thank the Netherlands Organisation for Scientific Research (NWO; an ECHO grant and a VIDI grant) for funding.
Author information
Authors and Affiliations
Contributions
J.M.P., J.B., A.B. and A.G.L.O. performed the experiments and analyzed the data, R.E. and J.H.v.E. designed the experiments, J.M.P. and R.E. wrote the paper, and all authors contributed to discussing the results and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Acid-catalyzed hydrogel formation.
Formation of a hydrazone-based hydrogel under acid-catalyzed conditions, by mixing buffered stock solutions of aldehyde and hydrazide at room temperature. (MOV 9170 kb)
Rights and permissions
About this article
Cite this article
Poolman, J., Boekhoven, J., Besselink, A. et al. Variable gelation time and stiffness of low-molecular-weight hydrogels through catalytic control over self-assembly. Nat Protoc 9, 977–988 (2014). https://doi.org/10.1038/nprot.2014.055
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.055
This article is cited by
-
Fluorescence microscopic visualization of functionalized hydrogels
NPG Asia Materials (2022)
-
Fabrication of alginate-based hydrogel cross-linked via horseradish peroxidase for articular cartilage engineering
BMC Research Notes (2021)
-
Hofmeister Anion-Induced Tunable Rheology of Self-Healing Supramolecular Hydrogels
Nanoscale Research Letters (2019)
-
Chemical signal activation of an organocatalyst enables control over soft material formation
Nature Communications (2017)
-
Free-standing supramolecular hydrogel objects by reaction-diffusion
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.