Abstract
Methamphetamine (METH)-induced neurotoxicity results in long-lasting depletions of monoamines and changes in basal ganglia function. We previously reported that rats with METH-induced neurotoxicity no longer engage dorsomedial striatum during a response-reversal learning task, as their performance is insensitive to acute disruption of dorsomedial striatal function by local infusion of an N-methyl-D-aspartate receptor antagonist or an antisense oligonucleotide against the activity-regulated cytoskeleton-associated (Arc) gene. However, METH-pretreated rats perform the task as well as controls. Therefore, we hypothesized that the neural circuitry involved in the learning had changed in METH-pretreated rats. To test this hypothesis, rats were pretreated with a neurotoxic regimen of METH or with saline. After 3–5 weeks, rats were trained on the reversal-learning task and in situ hybridization for Arc was performed. A significant correlation between Arc expression and performance on the task was found in nucleus accumbens shell of METH-, but not saline-, pretreated rats. Consistent with the idea that the correlation between Arc expression in a brain region and behavioral performance implicates that brain region in the learning, infusion of an antisense oligonucleotide against Arc into the shell impaired consolidation of reversal learning in METH-, but not saline-, pretreated rats. These findings provide novel evidence suggesting that METH-induced neurotoxicity leads to a shift from dorsal to ventral striatal involvement in the reversal-learning task. Such reorganization of neural circuitry underlying learning and memory processes may contribute to impaired cognitive function in individuals with METH-induced neurotoxicity or others with striatal dopamine loss, such as patients with Parkinson’s disease.
Similar content being viewed by others
INTRODUCTION
Methamphetamine (METH) abuse continues to have considerable societal impact, with 12 million Americans reporting use in their lifetime (2011 National Survey on Drug Use and Health, SAMHSA). METH abuse in humans causes decreases in the dopamine transporter (DAT; Wilson et al, 1996) and serotonin transporter (SERT; Sekine et al, 2006). Further, recent data indicate that people with a history of hospitalization for METH abuse are at higher risk of developing Parkinson’s disease (Callaghan et al, 2012).
The monoamine loss resulting from METH abuse in humans can be recapitulated in rodents. METH-induced neurotoxicity causes partial depletions of dopamine (DA) and serotonin (5-HT) (Wagner et al, 1980). As in human METH abusers (Dean et al, 2013), this partial monoamine loss is associated with cognitive deficits, including impairments in odor and object recognition, attentional set-shifting (Marshall and O'Dell, 2012), sequential motor learning (Chapman et al, 2001; Daberkow et al, 2005), formation of stimulus–response associations (Son et al, 2011) and inhibitory control over behavior (Son et al, 2013). The deficits in basal ganglia-mediated behaviors may arise secondary to impaired phasic DA neurotransmission in the partially denervated striatum (Howard et al, 2011, 2013a).
Arc (activity-regulated cytoskeleton-associated gene) is an effector immediate-early gene involved in synaptic plasticity and memory consolidation (Shepherd and Bear, 2011). Hippocampal Arc expression correlates with performance and is necessary for memory consolidation on the spatial version of the Morris water maze (Guzowski et al, 2000,2001). Similarly, our lab has reported correlations between Arc mRNA in dorsomedial striatum (DMS) and performance on a striatally mediated response-reversal learning task in normal rats (Daberkow et al, 2007, 2008), but not METH-pretreated rats (Daberkow et al, 2008), suggesting that although METH-pretreated rats perform as well as normal rats on the reversal-learning task, they may rely on different brain circuitry to perform the task (Daberkow et al, 2008; Pastuzyn et al, 2012). To test this hypothesis, we looked for correlations between Arc mRNA expression in different brain regions and reversal learning in METH-pretreated rats relative to controls. We found a significant correlation in the nucleus accumbens (NAc) shell in METH-pretreated rats that did not exist in saline-pretreated rats. Further, disruption of Arc signaling in the NAc shell of METH-, but not saline-, pretreated rats impaired consolidation of the reversal learning. Taken together with our previously published observations (Pastuzyn et al, 2012), these data suggest that METH-induced neurotoxicity is associated with reorganization of neural circuitry engaged in a learning and memory task typically dependent on DMS, and that correlations between Arc mRNA expression in brain regions and behavioral performance may be a viable ex vivo approach for mapping neural circuitry engaged in learning and memory tasks.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles River Laboratories, Raleigh, NC, USA; 275–300 g) were singly housed in tub cages on a 12 : 12-h light cycle. Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Utah and followed the Guide for the Care and Use of Laboratory Animals.
METH Pretreatment
Rats were treated with a neurotoxic regimen of (±)-METH-HCl (4 × 10 mg/kg, free base, at 2-h intervals, s.c.; NIDA, Research Triangle Park, NC, USA) over the course of 1 day as previously described (Daberkow et al, 2008; Son et al, 2013). The day after treatment, rats were returned to their home cages and given free access to food and water until training began.
DA and SERT Autoradiography
DAT and SERT autoradiography was performed as detailed previously (Barker-Haliski et al, 2012a; Boja et al, 1992; Pastuzyn et al, 2012; Son et al, 2013). For striatal sections, the buffer contained fluoxetine (Sigma-Aldrich, St Louis, MO, USA) to block binding to the SERT, whereas for prefrontal cortical (PFC) sections, fluoxetine was omitted from the buffer, as PFC sections incubated in buffer containing fluoxetine showed no staining (data not shown). Slides were apposed to film (Kodak Biomax MR film; Eastman Kodak, Rochester, NY, USA) for 24 h.
Arc Correlations with Response-Reversal Learning
Reversal-learning task
Response-reversal learning on a T-maze was conducted as previously described (Barker-Haliski et al, 2012b; Daberkow et al, 2007; Pastuzyn et al, 2012). Beginning 3 weeks after METH pretreatment, rats (METH-pretreated, n=9; saline-pretreated, n=10) were food restricted and habituated to the food reward and maze. The turn bias of each rat was determined, followed by acquisition training for 3 days and then reversal learning. During reversal learning, rats had to turn in the opposite direction from acquisition to receive the reward. The criterion for learning on both acquisition and reversal tasks was 9/10 correct turns in a row. Five minutes after reaching criterion on reversal, rats were exposed to CO2 for 1 min and then sacrificed by decapitation. Brains were quickly removed, flash frozen in 2-methylbutane (Mallinckrodt Baker, Phillipsburg, NJ, USA) on dry ice, and stored at −80 °C until sectioning.
Radioactive in situ hybridization histochemistry
Frozen brains were sectioned (12-μm; Cryocut 1800; Leica, Wetzlar, Germany). Sections from PFC (mm from Bregma: +3.7 to +2.2), striatum (+1.6 to −0.92 mm) and dorsal hippocampus (−2.3 to −3.6 mm) were thaw-mounted onto Superfrost Plus slides (VWR, Aurora, CO, USA) and stored at −20 °C. Infusion cannula placements were determined by eye at this time and recorded on schematic diagrams from a rat brain atlas (Paxinos and Watson, 1998).
To assess Arc mRNA expression, slides containing striatal, PFC, or hippocampal sections were post-fixed and delipidated as previously described (Ganguly and Keefe, 2001). Detection of Arc mRNA was accomplished using a full-length ribonucleotide probe (Barker-Haliski et al, 2012a; Daberkow et al, 2007, 2008; Howard et al, 2013b). The plasmid containing the cDNA for Arc (Lyford et al, 1995) was linearized with EcoRI. The antisense ribonucleotide probe was transcribed using 35S-UTP (striatum) or 33P-UTP (PFC and hippocampus; PerkinElmer, Waltham, MA, USA) and T7 RNA polymerase (Roche, Indianapolis, IN, USA). Radioactive in situ hybridization was performed as previously described, with slightly modified final washing procedures (Ganguly and Keefe, 2001). Slides were apposed to film (Biomax MR) for 4–6 days.
Image analysis
Densitometric analysis of digitized film images was conducted using NIH ImageJ software, yielding background-subtracted average gray values in several brain regions from one hemisphere of each of the four sections on the slide. The regions analyzed were: cingulate (Cg1), prelimbic (PLC), infralimbic (ILC), ventral orbitofrontal (vOFC) and lateral orbitofrontal (lOFC) cortices; DMS and dorsolateral striatum (DLS) and NAc core and shell; CA1, CA3, upper and lower blades of dentate gyrus (DG), and hilus of the dorsal hippocampus. For cortical regions, all cortical layers were analyzed.
Effect of Arc Antisense Oligonucleotide Infusion in NAc Shell
Two weeks after saline (n=11) or METH (n=13) pretreatment, rats were anesthetized with ketamine/xylazine (90/10 mg/kg, i.p.) and placed in a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). A dual 26-gauge guide cannula (Plastics One, Roanoke, VA, USA) was lowered to end bilaterally just dorsal to NAc shell (mm from Bregma: +2.2 AP; ±1.0 ML; −6.4 DV) and secured. Bilateral infusions into NAc shell during behavioral experiments were made through 33-gauge infusion cannulae extending 1.1 mm beyond the end of the guides into NAc shell.
The reversal task was the same as described above, except that 2 h before undergoing reversal learning, either an Arc antisense oligonucleotide, Arc nonsense oligonucleotide, or 0.1 M PBS (vehicle) was infused into NAc shell. The Arc antisense and nonsense oligonucleotides were prepared and infused (1 μl of oligonucleotide; 1 nmol/μl in 0.1 M PBS, pH 7.4) at 0.33 μl/min bilaterally into NAc shell, as previously described for DMS (Pastuzyn et al, 2012). The design of the oligonucleotides was based on the prior work of Guzowski et al (2000). Further, the concentration of oligonucleotide and volumes and rates of infusion were also based on that work, as well as that of other labs showing restricted delivery of the antisense oligonucleotide to specific brain regions, including the NAc core, lateral amygdala and anterior cingulate cortex (Holloway and McIntyre, 2011; Lv et al, 2011; Ploski et al, 2008). Post-infusion, rats rested in their home cages for 2 h, were trained to criterion (9/10 correct trials) on reversal, and were returned to their home cages overnight. ‘Reversal retention’ occurred 24 h later, during which rats were rewarded for turning in the reversal direction learned the previous day, until criterion (9/10 correct trials) was reached. No further infusions were made on the reversal-retention day.
Statistical Analysis
Unpaired t-tests were used to compare RTI-55 autoradiographic signals and trials to criterion on the reversal-learning task for the saline and METH pretreatment groups on which ex vivo analysis of Arc mRNA expression was completed. Trials to criterion on the reversal-learning task were also correlated with Arc mRNA expression. A two-factor MANOVA (pretreatment × day) followed by post hoc analysis with paired t-tests across acquisition days was used to assess any effect of METH pretreatment on trials to criterion on the 3 days of response acquisition. A two-way ANOVA was used to evaluate the effects of pretreatment (saline or METH) and treatment (infusion of Arc antisense, Arc nonsense, or PBS) on trials to criterion on the reversal-learning and reversal-retention tasks. All statistical tests were run using JMP v.9.0 (SAS Institute, Cary, NC, USA).
RESULTS
DAT and SERT Autoradiography
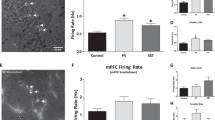
The administration of METH resulted in significant reductions in striatal and NAc DAT binding (Figure 1a). DAT depletions did not differ between rats used for ex vivo analysis of Arc mRNA expression and those used to examine the effects of Arc antisense oligonucleotide infusion into NAc shell, as two-way ANOVA revealed no main effect of ‘group’ (ex vivo Arc or Arc antisense group: DMS, p=0.99; DLS, p=0.7; NAc core, p=0.97; NAc shell, p=0.5) and no group × pretreatment (METH or saline) interaction in DMS (p=0.8), DLS (p=0.9), NAc core (p=0.97) or NAc shell (p=0.5). There were main effects of pretreatment in all four striatal regions. Unpaired, one-tailed t-tests revealed a significant decrease in DAT binding in rats pretreated with METH in DMS (t=12.1, p=0.0001), DLS (t=9.2, p=0.0001), NAc core (t=5.0, p=0.0001) and NAc shell (t=3.3, p=0.002).
METH neurotoxicity results in decreases in DAT and SERT binding. Graphs showing METH-induced decreases in (a) striatal DAT and (b) prefrontal cortical SERT as revealed by [125I]RTI-55 binding. As DAT and SERT binding between rats in the Arc correlation experiment and Arc antisense infusion experiment were not significantly different, binding values from both sets of rats are combined into one graph. Saline (SAL)-pretreated, n=21; METH-pretreated, n=22. *Significantly different from SAL, p<0.001.
Pretreatment with the binge regimen of METH also resulted in reductions in SERT binding in PFC (Figure 1b). As with DAT, SERT depletions were not significantly different between the two groups of rats, so the data were collapsed for the purpose of this analysis (no effect of group or group × pretreatment interaction, respectively, in Cg1 (p=0.5; p=0.5), PLC (p=0.3; p=0.3), ILC (p=0.4; p=0.4), vOFC (p=0.6; p=0.6) or lOFC (p=0.4; p=0.4)). Unpaired, one-tailed t-tests revealed significant decreases in SERT binding in all regions of PFC examined in METH-pretreated rats: Cg1, t=9.1, p=0.0001; PLC, t=6.1, p=0.0001; ILC, t=3.96, p=0.0003; vOFC, t=10.4, p=0.0001; lOFC, t=8.8, p=0.0001.
Effect of METH-Induced Neurotoxicity on Trials to Criterion for Acquisition and Reversal of Response Learning
As previously reported (Daberkow et al, 2008; Pastuzyn et al, 2012), there was no effect of METH pretreatment on acquisition of the response-learning task (F(1, 17)=0.2, p=0.6) and no pretreatment × acquisition day interaction (F(2, 16)=0.3, p=0.7). There was a main effect of acquisition day (F(2, 16)=8.4, p=0.03), with the rats overall taking significantly fewer trials to reach criterion on the third day of acquisition relative to both the second (t=−3.2, p=0.003) and first (t=−3.7, p=0.0008) days (data not shown). Rats with METH-induced monoamine depletions also did not differ from saline-pretreated controls in the numbers of trials to criterion on the reversal day (t=−0.9, p=0.4; data not shown).
Arc mRNA Expression
Analysis of the film autoradiograms revealed no significant differences between the levels of Arc mRNA expression in METH- vs saline-pretreated rats in DMS (t=0.2, p=0.8), DLS (t=0.2, p=0.8), NAc core (t=0.5, p=0.6) or NAc shell (t=1.6, p=0.1).
As in striatum, there was no significant effect of METH pretreatment on the levels of Arc mRNA expression as reflected in the radioactive in situ hybridization signal in Cg1 (t=−1.1, p=0.3), PLC (t=−1.2, p=0.2), ILC (t=0.2, p=0.8), vOFC (t=−0.9, p=0.4) or lOFC (t=−0.8, p=0.4). In hippocampal subregions, there were also no significant differences between the intensity of the Arc mRNA signals in the METH- vs the saline-pretreated rats in CA1 (t=−1.6, p=0.1), upper blade of the DG (t=−1.5, p=0.1) and lower blade of the DG (t=1.1, p=0.3). However, the intensity of the Arc mRNA in situ hybridization signal was significantly greater in METH- vs saline-pretreated rats in CA3 (t=−2.5, p=0.02) and hilus (t=−4.3, p=0.0007).
Previous work suggests that although Arc mRNA expression is induced in multiple brain regions in animals learning a particular behavior, the degree of that induction in a given brain area only correlates with measures of learning if that brain area is involved in the learning (Daberkow et al, 2007; Guzowski et al, 2001). Consequently, we did not include a caged control group in the present studies, because our prior work showed that reversal learning induces Arc throughout the brain (Daberkow et al, 2007), and we were testing whether there was a correlation between Arc in various brain regions and behavior, not whether there simply was an induction of Arc. Furthermore, our prior work suggests that rats with METH-induced monoamine depletions no longer rely on ‘normal’ striatal circuitry for response-reversal learning (Daberkow et al, 2008; Pastuzyn et al, 2012). Therefore, we used ex vivo analysis of Arc mRNA expression across multiple brain regions that might be involved in reversal learning in an attempt to reveal neural substrates being used by the METH-pretreated rats as they learned the reversal response.
Although PFC has been implicated in reversal learning (for review, see Ragozzino, 2007), we found no significant correlations in either saline- or METH-pretreated rats between Arc mRNA expression in PFC regions and trials to criterion on the reversal-learning task (Table 1). We further speculated that the METH-pretreated rats might be relying on a spatial strategy to solve the reversal task, and thus looked for correlations between Arc mRNA expression in hippocampal subregions and trials to criterion. Again, no significant correlations were found in either saline- or METH-pretreated rats (Table 1).
In contrast to the lack of significant correlations in the PFC and hippocampus, significant correlations were apparent in striatum, and the region in which the correlations were observed varied as a function of METH pretreatment. As previously reported (Daberkow et al, 2007, 2008), Arc mRNA expression in DMS, but not DLS, was significantly negatively correlated with trials to criterion on the reversal-learning task in saline-pretreated rats (Figure 2). No such significant correlation (p=0.9) was apparent for DMS of METH-pretreated rats, again consistent with our prior observations (Daberkow et al, 2008). However, in NAc shell, there was a significant negative correlation between Arc mRNA expression and performance in METH-pretreated rats (R2=0.44, p=0.0497) that was not apparent in saline-pretreated rats (p=0.2). Thus, prior exposure to a neurotoxic regimen of METH is associated with a change in the brain regions in which Arc mRNA expression correlates with behavioral performance, suggesting that the METH-pretreated rats might be relying on NAc shell, rather than DM striatum, in this task.
Correlations between Arc mRNA in striatal subregions and trials to criterion on the response-reversal learning task. Arc mRNA expression was determined by densitometric analysis of film autoradiograms using ImageJ and is expressed as background-subtracted average gray values (arbitrary units). Significant correlations (as indicated by box around R2 and p-values) were in DM striatum of saline (SAL)-pretreated rats (R2=0.56, p=0.013) and NAc shell of methamphetamine (METH)-pretreated rats (R2=0.44, p=0.0497). METH-pretreated rats were given a neurotoxic regimen of (±)-METH•HCl (4 × 10 mg/kg free base, s.c., at 2-h intervals) approximately 7 weeks before reversal learning. DLS, dorsolateral striatum; DMS, dorsomedial striatum; NAcC, nucleus accumbens core; NAcSh, nucleus accumbens shell.
Effect of Arc Antisense on Response-Reversal Learning and its Retention
Prior work from our lab (Pastuzyn et al, 2012) and others (Czerniawski et al, 2011; Guzowski et al, 2000; Hearing et al, 2011; Holloway and McIntyre, 2011; Maddox and Schafe, 2011; Ploski et al, 2008) has shown that disruption of Arc in a brain area known to be involved in completion of a particular learning/memory task disrupts consolidation of the memory. Thus, to further examine whether the circuitry mediating response-reversal learning and consolidation of that learning in METH-pretreated rats had shifted to rely on NAc shell, we determined whether local infusion of an Arc antisense oligonucleotide into NAc shell had differential effects on retention of the reversal learning in METH- vs saline-pretreated rats. Figure 3 illustrates the locations of the tips of the infusion cannulae in NAc shell for each rat.
Diagrams (Paxinos and Watson, 1998) showing placement of infusion sites (black dots) in the NAc shell of rats infused with an Arc antisense or Arc nonsense oligonucleotide or with PBS. Numbers indicate mm from Bregma.
Consistent with our prior observations (Daberkow et al, 2008; Pastuzyn et al, 2012), METH- and saline-pretreated rats did not differ in trials to criterion during the acquisition days (data not shown). Infusion of an Arc antisense oligonucleotide did not alter performance on the day of reversal learning (Figure 4a), as a two-way ANOVA on pretreatment (saline, METH) × treatment (Arc antisense, Arc nonsense, PBS) for trials to reach criterion on the reversal task revealed no significant main effect of pretreatment (F(1, 1)=0.05, p=0.8) or infusion (F(2, 2)=1.0, p=0.4) and no significant interaction (F(2, 2)=0.1, p=0.9).
Knockdown of Arc in NAc shell impairs consolidation of reversal learning in METH-, but not saline-, pretreated rats. Rats were infused with an Arc antisense oligonucleotide, Arc nonsense oligonucleotide, or PBS into NAc shell 2 h before response-reversal learning on a T-maze. (a) None of the compounds had any effect on reversal learning in saline- or METH-pretreated rats. (b) Rats were tested on reversal retention 24 h after reversal learning. Knockdown of Arc mRNA in NAc shell via an Arc antisense oligonucleotide impaired reversal retention in METH-, but not saline-, pretreated rats. Values are average trials to criterion (9/10 correct consecutive trials; ±SEM, n=3–6 per group) on the reversal-learning task (a) or on the reversal-retention test 24 h later (b). *Significantly different from all other groups, all p-values<0.05.
Rats were tested for retention of the reversal learning 24 h later. Two-way ANOVA on pretreatment × treatment for trials needed to reach criterion on the reversal-retention test revealed a main effect of pretreatment (Figure 4b; F(1, 18)=5.29, p=0.03), a trend toward an effect of treatment (F(2, 18)=2.81, p=0.09), and a significant pretreatment × treatment interaction (F(2, 2)=6.2, p=0.009). Tukey HSD post hoc analysis of the significant interaction revealed that infusion of Arc antisense into NAc shell during the reversal learning did not impair retention of the reversal learning in the saline-pretreated rats, as the trials to criterion on the retention day were not different from those in the saline-pretreated rats infused with a nonsense oligonucleotide (p=0.97) or PBS (p=0.995). Conversely, infusion of the Arc antisense oligonucleotide into NAc shell did impair retention of reversal learning in the METH-pretreated rats. METH-pretreated rats infused with the Arc antisense oligonucleotide during reversal learning took significantly more trials to reach criterion on the retention test the following day relative to METH-pretreated rats infused with the nonsense oligonucleotide (p=0.01) or PBS (p=0.03), as well as relative to the saline-pretreated rats infused with the Arc antisense oligonucleotide (p=0.003). Taken together with our prior results showing that infusion of Arc antisense into DMS impairs retention of reversal learning in saline-, but not METH-, pretreated rats (Pastuzyn et al, 2012), the accumulating evidence suggests that the neural circuitry in which consolidation of reversal learning occurs is altered as a consequence of METH-induced neurotoxicity.
DISCUSSION
Previous results suggest that the correlation between Arc mRNA expression in a brain region and behavioral performance on a task, rather than the simple presence of gene expression, reflects the necessity of synaptic modifications in that brain region for learning and its consolidation (Daberkow et al, 2007; Guzowski et al, 2001; Hearing et al, 2011; Pastuzyn et al, 2012). The present findings provide additional support for this view by showing again that disruption of Arc in a brain region impairs consolidation of learning only if a significant correlation between Arc expression in that brain region and behavioral performance was observed. The present work also confirms earlier results showing a loss of the normal correlation between Arc mRNA expression in DMS and response-reversal learning in rats with METH-induced neurotoxicity (Daberkow et al, 2008). This study extends those findings by demonstrating the appearance of a novel correlation between Arc expression in NAc shell and behavioral performance as a consequence of prior METH exposure and subsequent sensitivity of reversal-learning consolidation to infusion of an Arc antisense oligonucleotide into NAc shell. These findings suggest that prior neural injury—in this case, METH-induced neurotoxicity—leads to alterations in the neural circuitry engaged when an animal performs a learning and memory task, and that this change in circuitry can be monitored by evaluating the correlation between Arc mRNA expression in various brain regions and behavioral performance. This approach may therefore serve as an ex vivo imaging approach to interrogate neural circuits engaged in learning and memory tasks and how those circuits are affected by CNS insult.
The reversal learning examined in this study is typically dependent on the functional integrity of DMS, as infusion of an NMDA receptor antagonist or an antisense oligonucleotide against Arc into DMS in normal animals disrupts learning and consolidation of that learning, respectively (Palencia and Ragozzino, 2004; Pastuzyn et al, 2012). Despite this apparent specific role of DMS in response-reversal learning, in situ hybridization histochemical staining revealed expression of Arc mRNA throughout the brain. Thus, as previously suggested (Daberkow et al, 2007, 2008; Guzowski et al, 2001), evidence is accumulating that it is not simply the presence of Arc mRNA induction in a brain region that implicates plasticity processes in that region in the learning/memory formation; rather, it appears to be the correlation between Arc and the measure of learning that is the hallmark implicating synaptic plasticity processes in a brain region as being critical for the particular learning being examined and its consolidation.
Evidence that the correlation between Arc mRNA expression in a brain region and behavioral performance is the critical dependent measure for using Arc mRNA expression to identify brain regions involved in the learning/memory being examined comes from studies using site-specific infusions of antisense oligonucleotides to disrupt Arc function. For example, Guzowski et al (2001) reported a significant inverse correlation between hippocampal Arc expression and latency to escape in a spatial, but not cued, version of the Morris water maze. Antisense-mediated knockdown of Arc in hippocampus during learning on the spatial task impaired memory consolidation, as evidenced by impaired retention of the previously learned spatial location (Guzowski et al, 2000). Similarly, prior work by Hearing et al (2008) revealed a significant correlation between Arc mRNA expression in DLS and context-induced lever pressing (cocaine seeking) during a 1-h extinction test. Infusion of an Arc antisense oligonucleotide during that 1-h extinction session impaired consolidation, as evidenced by greater lever pressing in the antisense-infused rats when assessed 24 and 48 h later (Hearing et al, 2011). We also previously reported that normal rats show a significant inverse correlation between Arc mRNA in DMS and trials to criterion on the reversal-learning task (as reported herein and Daberkow et al, 2007, 2008) and that infusion of an Arc antisense oligonucleotide into DMS impairs consolidation of reversal learning in those normal animals (Pastuzyn et al, 2012). Importantly, in METH-pretreated rats the correlation between Arc mRNA expression in DMS and reversal learning is lost (as reported herein and Daberkow et al, 2007, 2008), and infusion of an Arc antisense oligonucleotide into the DMS does not impair consolidation of the reversal learning in these METH-pretreated rats (Pastuzyn et al, 2012). Similarly, in this study, infusion of the Arc antisense oligonucleotide into NAc shell of normal animals—a brain region in which Arc mRNA expression does not correlate with reversal learning—does not impair retention of the learned reversal, even though there is Arc expression in this region. Taken together, these findings suggest the interpretation that the correlation between Arc in a brain region and the index of learning can be used to map, ex vivo, the neural circuitry engaged in the particular learning and memory task.
We have previously reported that the correlation between Arc in DMS and reversal learning normally observed in intact rats is lacking in rats with METH-induced neurotoxicity, despite the fact that they have apparently normal response-reversal learning (Daberkow et al, 2008). Therefore, in the present work, we performed a broader evaluation of Arc mRNA in different brain regions and reversal learning in METH-pretreated rats. We discovered a novel correlation in METH-pretreated rats between Arc in NAc shell and reversal learning. In this case, infusion of Arc antisense into NAc shell during the reversal learning impaired consolidation of that learning. Although we did not directly verify that the antisense oligonucleotide-mediated knockdown of Arc remained confined to the NAc shell, two lines of evidence suggest that the effect of the antisense oligonucleotide observed in the METH-pretreated rats is likely due to loss of Arc function in the NAc shell. First, several prior studies have infused biotinylated Arc antisense oligonucleotides into specific brain regions similar in size to the NAc shell at concentrations, volumes and rates of infusion similar to those used here and have reported that the infused oligonucleotide remains restricted to the region in which it was infused (Holloway and McIntyre, 2011; Lv et al, 2011; Ploski et al, 2008). Second, although restriction of infused Arc antisense to the NAc shell has not been directly examined, the prior work by Lv et al (2011) reported dissociable effects of Arc antisense infusion into the NAc shell vs core on morphine-induced conditioned place preference. Taken together, these data suggest that infusion of Arc antisense oligonucleotide in this study likely specifically disrupted Arc function in the NAc shell, thereby disrupting reversal learning in the METH-pretreated rats.
The fact that METH-pretreated rats appear to rely on different striatal circuitry to perform the reversal task relative to intact controls is consistent with literature showing differences in neural circuitry activated during learning paradigms between normal individuals and individuals with CNS injury/disease, such as Parkinson’s disease (eg, Beauchamp et al, 2008; Moody et al, 2004; Rieckmann et al, 2010). For example, previous fMRI analysis of Parkinson’s disease patients who performed as well as controls on a probabilistic weather prediction task revealed that they activated medial temporal lobe during the task, whereas controls showed normal activation of basal ganglia circuitry (Moody et al, 2004). It is therefore critical to assess not just behavioral performance, but also the neural circuitry underlying behavioral performance, in order to fully appreciate the impact of CNS insult, as the neural circuitry mediating the behavior may be altered even if gross behavioral performance appears intact.
In the case of the present studies, the basis for this reorganization of task-related processing is unknown, but may be secondary to the METH-induced DA depletions. As confirmed in the present work, exposure to high doses of METH results in partial DA loss (Wagner et al, 1980). This loss is associated with impairment of phasic DA signaling (Howard et al, 2011, 2013a), along with loss of transcriptional activation and normal subcellular distribution of Arc mRNA in dorsal striatum (Barker-Haliski et al, 2012a), both of which are critical for synaptic plasticity underlying basal ganglia-mediated learning and memory processes (Calabresi et al, 2007; Schultz, 2007). As previously reported (eg, Haughey et al, 1999; Johnson-Davis et al, 2002; Ricaurte et al, 1980; Wallace et al, 1999), in this study, METH-induced DA loss in the NAc, particularly in the shell, was less extensive. Based on previous evidence from our lab, there appears to be a threshold (∼40% depletion) necessary for behavioral impairments to be evident (Daberkow et al, 2005). Perhaps the ∼25% depletion in NAc shell observed in this study was insufficient to prevent this brain region from being used by METH-pretreated rats in the behavioral task. Although we have observed disruption of DA transients in the NAc core of METH-pretreated rats (Howard et al, 2013a), whether there is less significant disruption of phasic DA signaling in NAc shell at these levels of METH-induced DA loss remains to be determined.
Both DMS and NAc shell are motor outputs for the basal ganglia, and NAc shell is also often touted as being involved in motivated and goal-directed behavior, (Humphries and Prescott, 2010; Ikemoto, 2007). Therefore, DA-mediated plasticity may be relatively preserved in NAc shell compared with DMS after METH-induced neurotoxicity, allowing DA-mediated synaptic modifications there to subserve consolidation of response-reversal learning. Furthermore, studies have shown that there are differences in how rostral and caudal NAc shell modulate behavior (Reynolds and Berridge, 2001). Our infusions targeted the rostral NAc shell, and given the differences in behavioral output of rostral and caudal NAc shell, as well as the anatomical inputs/outputs to the two regions (eg, Groenewegen et al, 1999; Usuda et al, 1998), it will be interesting in future studies to examine the relative contributions of the rostral vs caudal NAc shell to reversal learning in normal and METH-pretreated rats.
As noted above, the lack of effect of METH pretreatment on the levels of Arc mRNA expression in dorsal striatum in this study appears to be at odds with our prior work showing decreased Arc mRNA in such animals (Barker-Haliski et al, 2012a; Daberkow et al, 2008). This apparent difference likely arises from the approaches and, more so, the dependent measures, used in the different studies. In our former studies, we used fluorescent in situ hybridization (FISH) and determined the numbers of striatonigral vs striatopallidal neurons with Arc mRNA signal in different subcellular compartments. In this study, the radioactive in situ hybridization signal gives us a broad determination of Arc mRNA expression across both populations of striatal efferent neurons and in all subcellular compartments. Our work with FISH has shown that basal Arc mRNA transcription is increased in both striatal efferent neuron populations in rats with METH-induced neurotoxicity, but that the animals do not further induce Arc mRNA in response to behavioral activation (Barker-Haliski et al, 2012a; Daberkow et al, 2008). Further, METH-induced DA loss is associated with loss of Arc mRNA specifically in the cytoplasm of striatonigral efferent neurons (Barker-Haliski et al, 2012a; Daberkow et al, 2008). It is this latter effect that is apparent in our prior work (Barker-Haliski et al, 2012a; Daberkow et al, 2008), as the dependent measure reported is the number of neurons with Arc mRNA signal in the cytoplasm. In this study, because the radioactive in situ hybridization approach incorporates signal in both populations of neurons and in all subcellular compartments, the METH-induced loss of cytoplasmic Arc mRNA signal in one subpopulation of neurons is not apparent. That said, what the FISH and radioactive in situ approaches have in common is that both reveal the correlation between Arc mRNA expression and behavior (findings herein and in Daberkow et al, 2007, 2008).
The results of this study suggest that there is a change, following METH-induced neurotoxicity, in the brain circuitry used in a behavioral task. Further, they provide additional support for the proposition that a correlation between Arc mRNA expression in a brain region and the measure of learning on a task implicates synaptic plasticity processes in that brain region as being critical for learning and its consolidation. Since humans with a history of METH abuse also can have partial monoamine loss, they may be forced to rely on potentially ‘less ideal’ neural circuits to perform particular cognitive tasks, and this lack of normal cognitive processes may contribute to cognitive deficits seen (Dean et al, 2013), especially in more complicated tasks that may require engagement of several brain regions at once. Better understanding the impact of neurotoxicity on synaptic plasticity mechanisms should allow for the development of targeted therapies to address impaired cognitive function in individuals with METH-induced neurotoxicity or others with striatal DA loss, such as patients with Parkinson’s disease.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Barker-Haliski ML, Oldenburger K, Keefe KA (2012a). Disruption of subcellular Arc/Arg 3.1 mRNA expression in striatal efferent neurons following partial monoamine loss induced by methamphetamine. J Neurochem 123: 845–855.
Barker-Haliski ML, Pastuzyn ED, Keefe KA (2012b). Expression of the core exon-junction complex factor eukaryotic initiation factor 4A3 is increased during spatial exploration and striatally mediated learning. Neuroscience 226: 51–61.
Beauchamp MH, Dagher A, Panisset M, Doyon J (2008). Neural substrates of cognitive skill learning in Parkinson's disease. Brain Cogn 68: 134–143.
Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH et al (1992). High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse 12: 27–36.
Calabresi P, Picconi B, Tozzi A, Di Filippo M (2007). Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30: 211–219.
Callaghan RC, Cunningham JK, Sykes J, Kish SJ (2012). Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120: 35–40.
Chapman DE, Hanson GR, Kesner RP, Keefe KA (2001). Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther 296: 520–527.
Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA (2011). The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J Neurosci 31: 11200–11207.
Daberkow DP, Kesner RP, Keefe KA (2005). Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav 81: 198–204.
Daberkow DP, Riedy MD, Kesner RP, Keefe KA (2007). Arc mRNA induction in striatal efferent neurons associated with response learning. Eur J Neurosci 26: 228–241.
Daberkow DP, Riedy MD, Kesner RP, Keefe KA (2008). Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox Res 14: 307–315.
Dean AC, Groman SM, Morales AM, London ED (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38: 259–274.
Ganguly A, Keefe KA (2001). Unilateral dopamine depletion increases expression of the 2A subunit of the N-methyl-D-aspartate receptor in enkephalin-positive and enkephalin-negative neurons. Neuroscience 103: 405–412.
Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P (1999). Convergence and segregationg of ventral striatal inputs and outputs. Ann N Y Acad Sci 877: 49–63.
Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF et al (2000). Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20: 3993–4001.
Guzowski JF, Setlow B, Wagner EK, McGaugh JL (2001). Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci 21: 5089–5098.
Haughey HM, Fleckenstein AE, Hanson GR (1999). Differential regional effects of methampetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem 72: 661–668.
Hearing MC, Schwendt M, McGinty JF (2011). Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol 14: 784–795.
Hearing MC, See RE, McGinty JF (2008). Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struc Funct 213: 215–227.
Holloway CM, McIntyre CK (2011). Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol Learn Mem 95: 425–432.
Howard CD, Daberkow DP, Ramsson ES, Keefe KA, Garris PA (2013a). Methamphetamine-induced neurotoxicity disrupts naturally occurring phasic dopamine signaling. Eur J Neurosci 38: 2078–2088.
Howard CD, Keefe KA, Garris PA, Daberkow DP (2011). Methamphetamine-induced neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem 118: 668–676.
Howard CD, Pastuzyn ED, Barker-Haliski ML, Garris PA, Keefe KA (2013b). Phasic-like stimulation of the medial forebrain bundle augments striatal gene expression despite methamphetamine-induced partial dopamine denervation. J Neurochem 125: 555–565.
Humphries MD, Prescott TJ (2010). The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol 90: 385–417.
Ikemoto S (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27–78.
Johnson-Davis KL, Hanson GR, Keefe KA (2002). Long-term post-synaptic consequences of methamphetamine on preprotachykinin mRNA expression. J Neurochem 82: 1472–1479.
Lv X-F, Xu Y, Han J-S, Cui C-L (2011). Expression of activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) in the nucleus accumbens is critical for the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Behav Brain Res 223: 182–191.
Lyford GL, Yamagato K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG et al (1995). Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14: 433–445.
Maddox SA, Schafe GE (2011). The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci 31: 7073–7082.
Marshall JF, O’Dell SJ (2012). Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci 35: 536–545.
Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ (2004). An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci 118: 438–442.
Palencia CA, Ragozzino ME (2004). The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem 82: 81–89.
Pastuzyn ED, Chapman DE, Wilcox KS, Keefe KA (2012). Altered learning and Arc-regulated consolidation of learning in striatum by methamphetamine-induced neurotoxicity. Neuropsychopharmacology 37: 885–895.
Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press: Orlando, FL, USA.
Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA et al (2008). The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J Neurosci 28: 12383–12395.
Ragozzino ME (2007). The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann NY Acad Sci 1121: 355–375.
Reynolds SM, Berridge KC (2001). Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 21: 3261–3270.
Ricaurte GA, Schuster CR, Seiden LS (1980). Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193: 153–163.
Rieckmann A, Fischer H, Bäckman L (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. NeuroImage 50: 1303–1312.
SAMHAS (2011). National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. In: Results from the 2011 National Survey on Drug Use and Health Summary of National Findings. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. Substance Abuse and Mental Health Services Administration, 2012, Rockville, MD.
Schultz W (2007). Behavioral dopamine signals. Trends Neurosci 30: 203–210.
Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M et al (2006). Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63: 90–100.
Shepherd JD, Bear MF (2011). New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci 14: 279–284.
Son J-H, Kuhn J, Keefe KA (2013). Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology 67: 95–103.
Son J-H, Latimer C, Keefe KA (2011). Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology 36: 2441–2451.
Usuda I, Tanaka K, Chiba T (1998). Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 797: 73–93.
Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J (1980). Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res 181: 151–160.
Wallace TL, Gudelsky GA, Vorhees CV (1999). Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci 19: 9141–9148.
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996). Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Med 2: 699–703.
Acknowledgements
This work was supported by DA024036 (KAK) and DA032502 (EDP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pastuzyn, E., Keefe, K. Changes in Neural Circuitry Regulating Response-Reversal Learning and Arc-Mediated Consolidation of Learning in Rats with Methamphetamine-Induced Partial Monoamine Loss. Neuropsychopharmacol 39, 963–972 (2014). https://doi.org/10.1038/npp.2013.296
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.296