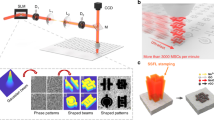
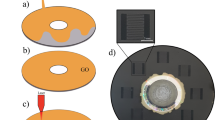
Abstract
Microscale supercapacitors provide an important complement to batteries in a variety of applications, including portable electronics. Although they can be manufactured using a number of printing and lithography techniques1,2,3, continued improvements in cost, scalability and form factor are required to realize their full potential. Here, we demonstrate the scalable fabrication of a new type of all-carbon, monolithic supercapacitor by laser reduction and patterning of graphite oxide films. We pattern both in-plane and conventional electrodes consisting of reduced graphite oxide with micrometre resolution, between which graphite oxide serves as a solid electrolyte4,5,6,7,8,9. The substantial amounts of trapped water in the graphite oxide makes it simultaneously a good ionic conductor and an electrical insulator, allowing it to serve as both an electrolyte and an electrode separator with ion transport characteristics similar to that observed for Nafion membranes10,11. The resulting micro-supercapacitor devices show good cyclic stability, and energy storage capacities comparable to existing thin-film supercapacitors1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chmiola, J., Largeot, C., Taberna, P. L., Simon, P. & Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 328, 480–483 (2010).
Pech, D. et al. Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J. Power Sources 195, 1266–1269 (2010).
Pech, D. et al. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nature Nanotech. 5, 651–654 (2010).
Liu, X. J. & Osaka, T. All-solid-state electric double-layer capacitor with isotropic high-density graphite electrode and polyethylene oxide/LiClO4 polymer electrolyte. J. Electrochem. Soc. 143, 3982–3986 (1996).
Rikukawa, M. & Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 25, 1463–1502 (2000).
Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958).
Gilje, S., Han, S., Wang, M., Wang, K. L. & Kaner, R. B. A chemical route to graphene for device applications. Nano Lett. 7, 3394–3398 (2007).
Gao, W., Alemany, L. B., Ci, L. J. & Ajayan, P. M. New insights into the structure and reduction of graphite oxide. Nature Chem. 1, 403–408 (2009).
Cerveny, S., Barroso-Bujans, F., Alegria, A. & Colmenero, J. Dynamics of water intercalated in graphite oxide. J. Phys. Chem. C 114, 2604–2612 (2010).
Thampan, T., Malhotra, S., Tang, H. & Datta, R. Modeling of conductive transport in proton-exchange membranes for fuel cells. J. Electrochem. Soc. 147, 3242–3250 (2000).
Park, K. W., Ahn, H. J. & Sung, Y. E. All-solid-state supercapacitor using a Nafion® polymer membrane and its hybridization with a direct methanol fuel cell. J. Power Sources 109, 500–506 (2002).
Eda, G., Fanchini, G. & Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nature Nanotech. 3, 270–274 (2008).
Tung, V. C., Allen, M. J., Yang, Y. & Kaner, R. B. High-throughput solution processing of large-scale graphene. Nature Nanotech. 4, 25–29 (2009).
Eda, G. & Chhowalla, M. Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv. Mater. 22, 2392–2415 (2010).
Cai, W. W. et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321, 1815–1817 (2008).
Casablanca, L. B. et al. NMR-based structural modeling of graphite oxide using multidimensional 13C solid-state NMR and ab initio chemical shift calculations. J. Am. Chem. Soc. 132, 5672–5676 (2010).
Park, S. et al. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 20, 6592–6594 (2008).
Wei, Z. et al. Nanoscale tunable reduction of graphene oxide for graphene electronics. Science 328, 1373–1375 (2010).
Zhang, Y. L. et al. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 5, 15–20 (2009).
Abraham, K. M., Jiang, Z. & Carroll, B. Highly conductive PEO-like polymer electrolytes. Chem. Mater. 9, 1978–1988 (1997).
Hirata, M., Gotou, T. & Ohba, M. Thin-film particles of graphite oxide. 2: Preliminary studies for internal micro fabrication of single particle and carbonaceous electronic circuits. Carbon 43, 503–510 (2005).
Petit, C., Seredych, M. & Bandosz, T. J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 19, 9176–9185 (2009).
Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462 (1995).
Saito, M., Arimura, N., Hayamizu, K. & Okada, T. Mechanisms of ion and water transport in perfluorosulfonated ionomer membranes for fuel cells. J. Phys. Chem. B 108, 16064–16070 (2004).
Mauritz, K. A. & Moore, R. B. State of understanding of Nafion. Chem. Rev. 104, 4535–4585 (2004).
Punckt, C., Pope, M. A., Liu, J., Lin, Y. & Aksay, L. A. Electrochemical performance of graphene as effected by electrode porosity and graphene functionalization. Electroanalysis 22, 2834–2841 (2010).
Taberna, P. L., Portet, C. & Simon, P. Electrode surface treatment and electrochemical impedance spectroscopy study on carbon/carbon supercapacitors. Appl. Phys. A 82, 639–646 (2006).
Balducci, A. et al. Cycling stability of a hybrid activated carbon/poly(3-methylthiophene) supercapacitor with N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquid as electrolyte. Electrochim. Acta 50, 2233–2237 (2005).
Chen, Y., Zhang, X., Zhang, D., Yu, P. & Ma, Y. High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes. Carbon 49, 573–580 (2011).
Stoller, M. D., Park, S. J., Zhu, Y. W., An, J. H. & Ruoff, R. S. Graphene-based ultracapacitors. Nano Lett. 8, 3498–3502 (2008).
Acknowledgements
W.G. and P.M.A. acknowledge funding support from Nanoholdings, LLC. The authors also thank B. Pradhan for discussions.
Author information
Authors and Affiliations
Contributions
W.G. prepared the GO, fabricated devices and collected characterization data. A.L.M.R., N.S. and W.G. conducted ionic conductivity testing and data analysis. Z.L., L.S. and W.G. conducted conductivity measurements and analysed the data. L.C. and R.V. were involved in study design. P.M.A., W.G. and L.C. designed the study. P.M.A., W.G. and N.S. wrote the paper. Q.Z. and B.W. conducted self-discharge measurements and analysed the relevant data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1370 kb)
Rights and permissions
About this article
Cite this article
Gao, W., Singh, N., Song, L. et al. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nature Nanotech 6, 496–500 (2011). https://doi.org/10.1038/nnano.2011.110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.110
This article is cited by
-
Ultraconformable Integrated Wireless Charging Micro-Supercapacitor Skin
Nano-Micro Letters (2024)
-
Enhancing supercapacitor performance through design optimization of laser-induced graphene and MWCNT coatings for flexible and portable energy storage
Scientific Reports (2023)
-
Machining water through laser cutting of nanoparticle-encased water pancakes
Nature Communications (2023)
-
Graphene oxide for photonics, electronics and optoelectronics
Nature Reviews Chemistry (2023)
-
Covalently anchored benzimidazole-reduced graphene oxide as efficient electrochemical supercapacitor electrode material
Journal of Materials Science: Materials in Electronics (2023)