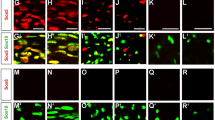
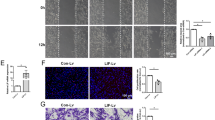
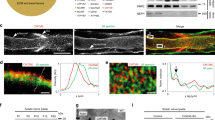
Abstract
Peripheral myelin formation is initiated by axonal cues that trigger a differentiation program in associated Schwann cells. Here, we define one essential differentiation signal: activation of the transcription factor NF-κB. In rat sciatic nerves, NF-κB was highly upregulated in pre-myelinating Schwann cells, and then its expression progressively declined until it was nearly absent in adults. Similarly, in co-cultures of Schwann cells and sensory neurons, NF-κB activation paralleled myelination, and blocking its activity or using cells from mice lacking the NF-κB subunit p65 markedly attenuated myelination. Inhibiting NF-κB also prevented activation of Oct-6, a transcription factor induced by axonal contact and required for proper myelin formation. These results show that the activation of NF-κB is an essential signal for the progression of axon-associated Schwann cells into a myelinating phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Monuki, E.S., Weinmaster, G., Kuhn, R. & Lemke, G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron 3, 783–793 (1989).
Arroyo, E.J., Bermingham, J.R. Jr., Rosenfeld, M.G. & Scherer, S.S. Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J. Neurosci. 18, 7891–7902 (1998).
Jaegle, M. et al. The POU factor Oct-6 and Schwann cell differentiation. Science 273, 507–510 (1996).
Bermingham, J.R. Jr. et al. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 10, 1751–1762 (1996).
Nagarajan, R. et al. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 30, 355–368 (2001).
Topilko, P. et al. Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796–799 (1994).
Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853–6866 (1999).
Chan, J.R., Cosgaya, J.M., Wu, Y.J. & Shooter, E.M. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 98, 14661–14668 (2001).
Haggiag, S. et al. Stimulation of myelin gene expression in vitro and of sciatic nerve remyelination by interleukin-6 receptor-interleukin-6 chimera. J. Neurosci. Res. 64, 564–574 (2001).
Xu, J., Zutter, M.M., Santoro, S.A. & Clark, R.A. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J. Cell. Biol. 140, 709–719 (1998).
Bushdid, P.B. et al. Inhibition of NF-kappaB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature 392, 615–618 (1998).
Mattson, M.P., Culmsee, C., Yu, Z. & Camandola, S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J. Neurochem. 74, 443–456 (2000).
Ghosh, S., May, M.J. & Kopp, E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
Einheber, S., Milner, T.A., Giancotti, F. & Salzer, J.L. Axonal regulation of Schwann cell integrin expression suggests a role for alpha 6 beta 4 in myelination. J. Cell Biol. 123, 1223–1236 (1993).
Scherer, S.S. The biology and pathobiology of Schwann cells. Curr. Opin. Neurol. 10, 386–397 (1997).
Kunsch, C., Ruben, S.M. & Rosen, C.A. Selection of optimal kappa B/Rel DNA-binding motifs: interaction of both subunits of NF-kappa B with DNA is required for transcriptional activation. Mol. Cell. Biol. 12, 4412–4421 (1992).
Lin, Y.Z., Yao, S.Y., Veach, R.A., Torgerson, T.R. & Hawiger, J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255–14258 (1995).
Feltri, M.L. et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199–209 (2002).
Mattson, M.P., Culmsee, C., Yu, Z. & Camandola, S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J. Neurochem. 74, 443–456 (2000).
Zorick, T.S., Syroid, D.E., Brown, A., Gridley, T. & Lemke, G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126, 1397–4406 (1999).
Lee, H.H., Dadgostar, H., Cheng, Q., Shu, J. & Cheng, G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in lymphocytes. Proc. Natl. Acad. Sci. USA 96, 9136–9141 (1999).
Chu, Z.L. et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl. Acad. Sci. USA 94, 10057–10062 (1997).
Guttridge, D.C., Albanese, C., Reuther, J.Y., Pestell, R.G. & Baldwin, A.S. Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19, 5785–5799 (1999).
Blanchard, A.D. et al. Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J. Neurosci. Res. 46, 630–640 (1996).
Huang, C. et al. Tumor necrosis factor modulates transcription of myelin basic protein gene through nuclear factor kappa B in a human oligodendroglioma cell line. Int. J. Dev. Neurosci. 20, 289–296 (2002).
Betz, U.A. et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic and pulmonary defects. J. Exp. Med. 188, 1955–1965 (1998).
Carter, B.D. et al. Selective activation of NFkB by nerve growth factor through the neurotrophin receptor p75. Science 272, 542–545 (1996).
Coetzee, T. et al. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86, 209–19 (1996).
Forghani, R., Nesbitt, J., Snipes, J., Shooter, E.M. & Peterson, A. Preparation of nuclear extracts from myelinating Schwann cells. J. Neurosci. Methods 89, 129–132 (1999).
Acknowledgements
The authors thank J.L. Salzer, S.O. Yoon and members of the Carter lab for helpful suggestions and the Vanderbilt Cell Imaging Core for technical support. We also are grateful to Regeneron Corp. for providing the neurotrophins. This work was supported by a NIH grant (NS38220), a Christopher Reeve Paralysis Foundation grant (CAC1-9803-2) and a Wadsworth Foundation grant to B.D.C., a NIH training grant (MH19732) to J.C.N. and a NIH grant (ES06387) to W.V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nickols, J., Valentine, W., Kanwal, S. et al. Activation of the transcription factor NF-κB in Schwann cells is required for peripheral myelin formation. Nat Neurosci 6, 161–167 (2003). https://doi.org/10.1038/nn995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn995
This article is cited by
-
Inhibition of RIPK1 by ZJU-37 promotes oligodendrocyte progenitor proliferation and remyelination via NF-κB pathway
Cell Death Discovery (2022)
-
Vexed mutations promote degeneration of dopaminergic neurons through excessive activation of the innate immune response
npj Parkinson's Disease (2022)
-
Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder
Nature Biomedical Engineering (2019)
-
Reversal of proliferation deficits caused by chromosome 16p13.11 microduplication through targeting NFκB signaling: an integrated study of patient-derived neuronal precursor cells, cerebral organoids and in vivo brain imaging
Molecular Psychiatry (2019)
-
IKKβ-mediated inflammatory myeloid cell activation exacerbates experimental autoimmune encephalomyelitis by potentiating Th1/Th17 cell activation and compromising blood brain barrier
Molecular Neurodegeneration (2016)