Abstract
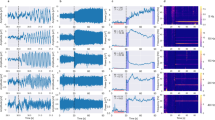
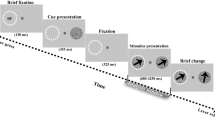
When attention is voluntarily directed to a spatial location, visual sensitivity increases at that location. What causes this improved sensitivity? Studies of single neuron spike rates in monkeys have provided mixed results in regard to whether attending to a stimulus increases its effective contrast (contrast gain) or multiplicatively boosts stimulus-driven neural responses (response or activity gain). We monitored frequency-tagged steady-state visual evoked potentials (SSVEPs) in humans and found that voluntary sustained attention multiplicatively increased stimulus-driven population electrophysiological activity. Analyses of intertrial phase coherence showed that this attentional response gain was at least partially due to the increased synchronization of SSVEPs to stimulus flicker. These results suggest that attention operates in a complementary manner at different levels; attention seems to increase single-neuron spike rates in a variety of ways, including contrast, response and activity gains, while also inducing a multiplicative boost on neural population activity via enhanced response synchronization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Reynolds, J.H., Pasternak, T. & Desimone, R. Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 (2000).
Di Russo, F., Spinelli, D. & Morrone, M.C. Automatic gain control contrast mechanisms are modulated by attention in humans: evidence from visual evoked potentials. Vision Res. 41, 2435–2447 (2001).
Cameron, E.L., Tai, J.C. & Carrasco, M. Covert attention affects the psychometric function of contrast sensitivity. Vision Res. 42, 949–967 (2002).
Ling, S. & Carrasco, M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 46, 1210–1220 (2006).
Huang, L. & Dobkins, K.R. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 45, 1201–1212 (2005).
Carrasco, M., Ling, S. & Read, S. Attention alters appearance. Nat. Neurosci. 7, 308–313 (2004).
Williford, T. & Maunsell, J.H. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 96, 40–54 (2006).
Morrone, M.C., Denti, V. & Spinelli, D. Color and luminance contrasts attract independent attention. Curr. Biol. 12, 1134–1137 (2002).
Lee, D.K., Itti, L., Koch, C. & Braun, J. Attention activates winner-take-all competition among visual filters. Nat. Neurosci. 2, 375–381 (1999).
Reynolds, J.H. & Chelazzi, L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 27, 611–647 (2004).
Martinez-Trujillo, J. & Treue, S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron 35, 365–370 (2002).
Fries, P., Reynolds, J.H., Rorie, A.E. & Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563 (2001).
Posner, M.I., Snyder, C.R. & Davidson, B.J. Attention and the detection of signals. J. Exp. Psychol. 109, 160–174 (1980).
Maunsell, J.H. & Cook, E.P. The role of attention in visual processing. Phil. Trans. R. Soc. Lond. B 357, 1063–1072 (2002).
Steinmetz, P.N. et al. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404, 187–190 (2000).
Azouz, R. & Gray, C.M. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc. Natl. Acad. Sci. USA 97, 8110–8115 (2000).
Niebur, E. & Koch, C. A model for the neuronal implementation of selective visual attention based on temporal correlation among neurons. J. Comput. Neurosci. 1, 141–158 (1994).
Buia, C. & Tiesinga, P. Attentional modulation of firing rate and synchrony in a model cortical network. J. Comput. Neurosci. 20, 247–264 (2006).
Henrie, J.A. & Shapley, R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J. Neurophysiol. 94, 479–490 (2005).
Regan, D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine (Elsevier, New York, 1989).
Morgan, S.T., Hansen, J.C. & Hillyard, S.A. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc. Natl. Acad. Sci. USA 93, 4770–4774 (1996).
Müller, M.M. et al. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Brain Res. Cogn. Brain Res. 6, 249–261 (1998).
Müller, M.M., Malinowski, P., Gruber, T. & Hillyard, S.A. Sustained division of the attentional spotlight. Nature 424, 309–312 (2003).
Ding, J., Sperling, G. & Srinivasan, R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb. Cortex 16, 1016–1029 (2005).
Fell, J., Fernandez, G., Klaver, P., Elger, C.E. & Fries, P. Is synchronized neuronal gamma activity relevant for selective attention? Brain Res. Brain Res. Rev. 42, 265–272 (2003).
Schiller, P.H., Logothetis, N.K. & Charles, E.R. Role of the color-opponent and broad-band channels in vision. Vis. Neurosci. 5, 321–346 (1990).
Levitt, J.B., Kiper, D.C. & Movshon, J.A. Receptive fields and functional architecture of macaque V2. J. Neurophysiol. 71, 2517–2542 (1994).
Hawken, M.J., Shapley, R.M. & Grosof, D.H. Temporal-frequency selectivity in monkey visual cortex. Vis. Neurosci. 13, 477–492 (1996).
Edwards, D.P., Purpura, K.P. & Kaplan, E. Contrast sensitivity and spatial frequency response of primate cortical neurons in and around the cytochrome oxidase blobs. Vision Res. 35, 1501–1523 (1995).
Varela, F., Lachaux, J.P., Rodriguez, E. & Martinerie, J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2001).
Geisler, W.S. & Albrecht, D.G. Visual cortex neurons in monkeys and cats: detection, discrimination, and identification. Vis. Neurosci. 14, 897–919 (1997).
Rager, G. & Singer, W. The response of cat visual cortex to flicker stimuli of variable frequency. Eur. J. Neurosci. 10, 1856–1877 (1998).
Srinivasan, R., Russell, D.P., Edelman, G.M. & Tononi, G. Increased synchronization of neuromagnetic responses during conscious perception. J. Neurosci. 19, 5435–5448 (1999).
Tallon-Baudry, C., Bertrand, O., Delpuech, C. & Pernier, J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 16, 4240–4249 (1996).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Reynolds, J.H., Chelazzi, L. & Desimone, R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 19, 1736–1753 (1999).
Chelazzi, L., Duncan, J., Miller, E.K. & Desimone, R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J. Neurophysiol. 80, 2918–2940 (1998).
Martinez-Conde, S., Macknik, S.L. & Hubel, D.H. The role of fixational eye movements in visual perception. Nat. Rev. Neurosci. 5, 229–240 (2004).
Kastner, S. & Ungerleider, L.G. The neural basis of biased competition in human visual cortex. Neuropsychologia 39, 1263–1276 (2001).
Mishkin, M., Ungerleider, L.G. & Macko, K.A. Object vision and spatial vision: two central pathways. Trends Neurosci. 6, 414–417 (1983).
Leopold, D.A. & Logothetis, N.K. Multistable phenomena: changing views in perception. Trends Cogn. Sci. 3, 254–264 (1999).
Grill-Spector, K. & Malach, R. The human visual cortex. Annu. Rev. Neurosci. 27, 649–677 (2004).
Sperling, G. & Melchner, M.J. The attention operating characteristic: examples from visual search. Science 202, 315–318 (1978).
Suzuki, S. Attention-dependent brief adaptation to contour orientation: a high-level aftereffect for convexity? Vision Res. 41, 3883–3902 (2001).
O'Craven, K.M., Rosen, B.R., Kwong, K.K., Treisman, A. & Savoy, R.L. Voluntary attention modulates fMRI activity in human MT-MST. Neuron 18, 591–598 (1997).
Treisman, A. & Sato, S. Conjunction search revisited. J. Exp. Psychol. Hum. Percept. Perform. 16, 459–478 (1990).
Luck, S.J. et al. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J. Exp. Psychol. Hum. Percept. Perform. 20, 887–904 (1994).
Campbell, F.W. & Maffei, L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J. Physiol. (Lond.) 207, 635–652 (1970).
Hou, C., Pettet, M.W., Sampath, V., Candy, T.R. & Norcia, A.M. Development of the spatial organization and dynamics of lateral interactions in the human visual system. J. Neurosci. 23, 8630–8640 (2003).
De Valois, R.L., Albrecht, D.G. & Thorell, L.G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 22, 545–559 (1982).
Acknowledgements
This work was supported by a US National Institutes of Health Grant EY14110 to S.S. and by National Institutes of Health Grant NS34639 and US National Science Foundation Grant BCS0518800 to K.A.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Joon Kim, Y., Grabowecky, M., Paller, K. et al. Attention induces synchronization-based response gain in steady-state visual evoked potentials. Nat Neurosci 10, 117–125 (2007). https://doi.org/10.1038/nn1821
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1821