Abstract
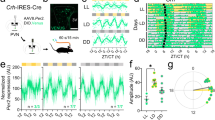
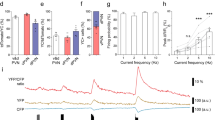
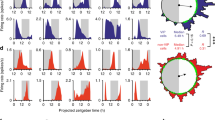
The suprachiasmatic nucleus (SCN) drives circadian rhythms of locomotor behavior by releasing factors that act on receptor sites near the third ventricle. Here we show that cardiotrophin-like cytokine (CLC) satisfies multiple criteria for a circadian regulator of locomotor activity. In the mouse, CLC is expressed in a subpopulation of SCN vasopressin neurons with a circadian rhythm that peaks during the daily period of locomotor quiescence. CLC receptors flank the third ventricle, and acute infusion of CLC into the third ventricle produced a transient blockade of locomotor activity without affecting the circadian clock. The hypothalamic infusion of neutralizing antibodies to the CLC receptor produced extra daily locomotor activity at the time when CLC is maximally expressed. These results suggest that CLC is probably an SCN output signal important for shaping daily rhythms of behavior; moreover, they indicate an unexpected role for a cytokine in adult brain function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
King, D.P. & Takahashi, J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 23, 713–742 (2000).
Pittendrigh, C.S. & Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. A 106, 223–252 (1976).
Naylor, E. et al. Effect of aging on sleep in the golden hamster. Sleep 21, 687–696 (1998).
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515 (2001).
LeSauter, J., Romero, P., Cascio, M. & Silver, R. Attachment site of grafted SCN influences precision of restored circadian rhythm. J. Biol. Rhythms 12, 327–338 (1997).
Silver, R., LeSauter, J., Tresco, P.A. & Lehman, M.N. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813 (1996).
Vogelbaum, M.A. & Menaker, M. Temporal chimeras produced by hypothalamic transplants. J. Neurosci. 12, 3619–3627 (1992).
Abe, M. et al. Circadian rhythms in isolated brain regions. J. Neurosci. 22, 350–356 (2002).
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Balsalobre, A. et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000).
Sujino, M. et al. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr. Biol. 13, 664–668 (2003).
Davis, F.C. & Menaker, M. Hamsters through time's window: temporal structure of hamster locomotor rhythmicity. Am. J. Physiol. 239, R149–R155 (1980).
Cheng, M.Y. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 (2002).
Kramer, A., Yang, F.-C., Kraves, S. & Weitz, C.J. A screen for secreted factors of the suprachiasmatic nucleus. Methods Enzymol. 393, 645–663 (2005).
Vallières, L. & Rivest, S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J. Neurochem. 69, 1668–1683 (1997).
Shi, Y. et al. Computational EST database analysis identifies a novel member of the neuropoietic cytokine family. Biochem. Biophys. Res. Commun. 262, 132–138 (1999).
Senaldi, G. et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc. Natl. Acad. Sci. USA 96, 11458–11463 (1999).
Muller-Newen, G. The cytokine receptor gp130: faithfully promiscuous. Sci. STKE 2003, PE40 (2003).
Derouet, D. et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc. Natl. Acad. Sci. USA 101, 4827–4832 (2004).
Fukada, T. et al. Signaling through Gp130: toward a general scenario of cytokine action. Growth Factors 17, 81–91 (1999).
Gekakis, N. et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998).
Bunger, M.K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Yoo, S.H. et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc. Natl. Acad. Sci. USA 102, 2608–2613 (2005).
Murre, C. et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58, 537–544 (1989).
Wang, Z., Ren, S.G. & Melmed, S. Hypothalamic and pituitary leukemia inhibitory factor gene expression in vivo: a novel endotoxin-inducible neuro-endocrine interface. Endocrinology 137, 2947–2953 (1996).
Watts, A.G. & Swanson, L.W. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 258, 230–252 (1987).
Schwartz, W.J., Gross, R.A. & Morton, M.T. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc. Natl. Acad. Sci. USA 84, 1694–1698 (1987).
Schobitz, B., de Kloet, E.R., Sutanto, W. & Holsboer, F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur. J. Neurosci. 5, 1426–1435 (1993).
Auernhammer, C.J. & Melmed, S. Interleukin-11 stimulates proopiomelanocortin gene expression and adrenocorticotropin secretion in corticotroph cells: evidence for a redundant cytokine network in the hypothalamo-pituitary-adrenal axis. Endocrinology 140, 1559–1566 (1999).
DeChiara, T.M. et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 83, 313–322 (1995).
Ware, C.B. et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development 121, 1283–1299 (1995).
Yoshida, K. et al. (1996). Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc. Natl. Acad. Sci. USA 93, 407–411 (1996).
Rhee, K.D., Goureau, O., Chen, S. & Yang, X.J. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J. Neurosci. 24, 9779–9788 (2004).
Palmqvist, P., Persson, E., Conaway, H.H. & Lerner, U.H. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J. Immunol. 169, 3353–3362 (2002).
Elson, G.C. et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat. Neurosci. 3, 867–872 (2000).
Watts, A.G., Swanson, L.W. & Sanchez-Watts, G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 258, 204–229 (1987).
Chesnokova, V. & Melmed, S. Minireview: neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology 143, 1571–1574 (2002).
Krueger, J.M. et al. The role of cytokines in physiological sleep regulation. Ann. NY Acad. Sci. 933, 211–221 (2001).
Rusak, B. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J. Comp. Physiol. A 118, 145–164 (1977).
Aton, S.J., Colwell, C.S., Harmar, A.J., Waschek, J. & Herzog, E.D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 8, 476–483 (2005).
Moore, R.Y., Speh, J.C. & Leak, R.K. Suprachiasmatic nucleus organization. Cell Tissue Res. 309, 89–98 (2002).
Stoleru, D., Peng, Y., Agosto, J. & Rosbash, M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868 (2004).
Grima, B., Chelot, E., Xia, R. & Rouyer, F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873 (2004).
Tautz, D. Segmentation. Dev. Cell 7, 301–312 (2004).
Houchmandzadeh, B., Wieschaus, E. & Leibler, S. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature 415, 798–802 (2002).
Acknowledgements
We thank M. Liu for technical assistance, L. Zakhary for advice on in situ hybridization and D. Knutti and K.-F. Storch for comments on the manuscript. Confocal images were obtained at the Harvard Center for Neurodegeneration and Repair. This work was supported by the US National Institute of Neurological Disorders and Stroke (C.J.W.), a Howard Hughes Medical Institute predoctoral fellowship (S.K.) and a Stuart H.Q. and Victoria Quan Fellowhip (S.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
In situ hybridization to coronal mouse brain sections showing constitutive expression of clc mRNA in the anterodorsal thalamus [Franklin, K. B. & Paxinos, G. The mouse brain in stereotaxic coordinates. (Academic Press, 1997)] over a circadian cycle. (PDF 152 kb)
Supplementary Fig. 2
Cells expressing clc mRNA are located in the dorsal division of the mouse SCN and distributed throughout its rostrocaudal axis. (PDF 114 kb)
Supplementary Fig. 3
Induction of clc mRNA by light in the mouse SCN. (PDF 111 kb)
Supplementary Fig. 4
Blockade of locomotor activity by activation of hypothalamic CLC receptors does not reflect a generalized inhibition of behavior. (PDF 79 kb)
Rights and permissions
About this article
Cite this article
Kraves, S., Weitz, C. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci 9, 212–219 (2006). https://doi.org/10.1038/nn1633
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1633
This article is cited by
-
A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus
Nature Communications (2023)
-
Identification of the suprachiasmatic nucleus venous portal system in the mammalian brain
Nature Communications (2021)
-
Vasopressin in circadian function of SCN
Journal of Biosciences (2020)
-
Circadian rhythms: a possible new player in non-alcoholic fatty liver disease pathophysiology
Journal of Molecular Medicine (2019)
-
The suprachiasmatic nucleus: age-related decline in biological rhythms
The Journal of Physiological Sciences (2016)