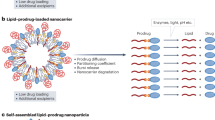
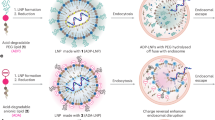
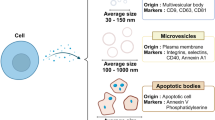
Abstract
A multitude of micro- and nanoparticles have been developed to improve the delivery of systemically administered pharmaceuticals, which are subject to a number of biological barriers that limit their optimal biodistribution. Bioinspired drug-delivery carriers formulated by bottom-up or top-down strategies have emerged as an alternative approach to evade the mononuclear phagocytic system and facilitate transport across the endothelial vessel wall. Here, we describe a method that leverages the advantages of bottom-up and top-down strategies to incorporate proteins derived from the leukocyte plasma membrane into lipid nanoparticles. The resulting proteolipid vesicles—which we refer to as leukosomes—retained the versatility and physicochemical properties typical of liposomal formulations, preferentially targeted inflamed vasculature, enabled the selective and effective delivery of dexamethasone to inflamed tissues, and reduced phlogosis in a localized model of inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Torchilin, V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nature Rev. Drug Discov. 13, 813–827 (2014).
Mitragotri, S., Burke, P. A. & Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature Rev. Drug Discov. 13, 655–672 (2014).
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotech. 3, 151–157 (2008).
Parodi, A. et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano 8, 9874–9883 (2014).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nature Mater. 12, 991–1003 (2013).
Kudgus, R. A. et al. Tuning pharmacokinetics and biodistribution of a targeted drug delivery system through incorporation of a passive targeting component. Sci. Rep. 4, 5669 (2014).
Luk, B. T. & Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Rel. 220, 600–607 (2015).
Hu, C.-M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Hu, C.-M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Parodi, A. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nature Nanotech. 8, 61–68 (2013).
Hammer, D. A. et al. Leuko-polymersomes. Faraday Discuss 139, 129–141 (2008).
Doshi, N. et al. Platelet mimetic particles for targeting thrombi in flowing blood. Adv. Mater. 24, 3864–3869 (2012).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnol. 33, 941–951 (2015).
Robbins, G. P. et al. Tunable leuko-polymersomes that adhere specifically to inflammatory markers. Langmuir 26, 14089–14096 (2010).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Toledano Furman, N. E. et al. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 13, 3248–3255 (2013).
Yoo, J-W., Irvine, D. J., Discher, D. E. & Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nature Rev. Drug Discov. 10, 521–535 (2011).
Alvarez-Lorenzo, C. & Concheiro, A. Bioinspired drug delivery systems. Curr. Opin. Biotechnol. 24, 1167–1173 (2013).
Gutiérrez Millán, C., Colino Gandarillas, C. I., Sayalero Marinero, M. L. & Lanao, J. M. Cell-based drug-delivery platforms. Ther. Deliver. 3, 25–41 (2012).
Millan, C. G., Marinero, M. A. L. S., Castaneda, A. Z. & Lanao, J. M. Drug, enzyme and peptide delivery using erythrocytes as carriers. J. Control. Release 95, 27–49 (2004).
Bretscher, M. S. Asymmetrical lipid bilayer structure for biological membranes. Nature 236, 11–12 (1972).
Demetzos, C. Differential scanning calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 18, 159–173 (2008).
Manconi, M. et al. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle–skin interaction. Eur. J. Pharm. Biopharm. 78, 27–35 (2011).
Mura, S., Manconi, M., Sinico, C., Valenti, D. & Fadda, A. M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Intl. J. Pharm. 380, 72–79 (2009).
Chow, T. S. Nanoscale surface roughness and particle adhesion on structured substrates. Nanotechnology 18, 115713 (2007).
Schaap, I. A., Eghiaian, F., des Georges, A. & Veigel, C. Effect of envelope proteins on the mechanical properties of influenza virus. J. Biol. Chem. 287, 41078–41088 (2012).
Mereghetti, P. et al. A Fourier transform infrared spectroscopy study of cell membrane domain modifications induced by docosahexaenoic acid. Biochim. Biophys. Acta 1840, 3115–3122 (2014).
Lodish, H. et al. Molecular Cell Biology (W. H. Freeman & Co., 2000).
Benmerah, A., Scott, M., Poupon, V. & Marullo, S. Nuclear functions for plasma membrane associated proteins? Traffic 4, 503–511 (2003).
Durr, E. et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nature Biotechnol. 22, 985–992 (2004).
Lund, R., Leth-Larsen, R., Jensen, O. N. & Ditzel, H. J. Efficient isolation and quantitative proteomic analysis of cancer cell plasma membrane proteins for identification of metastasis-associated cell surface markers. J. Proteome Res. 8, 3078–3090 (2009).
Liu, X., Zhang, M., Go, V. L. W. & Hu, S. Membrane proteomic analysis of pancreatic cancer cells. J. Biomed. Sci. 17, 74 (2010).
Corbo, C. et al. Proteomic profiling of a biomimetic drug delivery platform. Curr. Drug Targets 16, 1540–1547 (2015).
Zarbock, A., Ley, K., McEver, R. P. & Hidalgo, A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751 (2011).
Soto Pantoja, D. R., Kaur, S., Miller, T. W., Isenberg, J. S. & Roberts, D. D. Leukocyte surface antigen CD47. UCSD Mol. Pages 2, http://dx.doi.org/10.6072/H0.MP.A005186.01 (2013).
Hu, C.-M. J. et al. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5, 2664–2668 (2013).
Allen, T. Liposomal drug formulations. Drugs 56, 747–756 (1998).
Cosco, D., Paolino, D., Cilurzo, F., Casale, F. & Fresta, M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Intl. J. Pharm. 422, 229–237 (2012).
Bernsdorff, C., Reszka, R. & Winter, R. Interaction of the anticancer agent Taxol TM (paclitaxel) with phospholipid bilayers. J. Biomed. Mater. Res. 46, 141–149 (1999).
Franchimont, D., Kino, T., Galon, J., Meduri, G. U. & Chrousos, G. Glucocorticoids and inflammation revisited: the state of the art. Neuroimmunomodulation 10, 247–260 (2002).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nature Med. 15, 455–461 (2009).
Azzopardi, E. A., Ferguson, E. L. & Thomas, D. W. The enhanced permeability retention effect: a new paradigm for drug targeting in infection. J. Antimicrob. Chemother. 68, 257–274 (2013).
Ishibashi, M. et al. Integrin LFA-1 regulates cell adhesion via transient clutch formation. Biochem. Biophys. Res. Commun. 464, 459–466 (2015).
Arroyo, A. G. et al. Induction of tyrosine phosphorylation during ICAM-3 and LFA-1-mediated intercellular adhesion, and its regulation by the CD45 tyrosine phosphatase. J. Cell Biol. 126, 1277–1286 (1994).
Sigal, A. et al. The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment. J. Immunol. 165, 442–452 (2000).
Lorenz, H. M. et al. CD45 mAb induces cell adhesion in peripheral blood mononuclear cells via lymphocyte function-associated antigen-1 (LFA-1) and intercellular cell adhesion molecule 1 (ICAM-1). Cell. Immunol. 147, 110–128 (1993).
Chen, X. et al. Inflamed leukocyte-mimetic nanoparticles for molecular imaging of inflammation. Biomaterials 32, 7651–7661 (2011).
Sherman, M. B. et al. Removal of divalent cations induces structural transitions in red clover necrotic mosaic virus, revealing a potential mechanism for RNA release. J. Virol. 80, 10395–10406 (2006).
Chiappini, C. et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nature Mater. 14, 532–539 (2015).
Copp, J. A. et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl Acad. Sci. USA 111, 13481–13486 (2014).
Acknowledgements
The authors would like to gratefully acknowledge M. Ferrari for valuable and stimulating discussions about the study. The authors would like to thank J. You for his help in the animal procedures. The authors acknowledge support from the National Institute of Health (1R21CA173579-01A1 and 5U54CA143837 PSOC Pilot project), the Department of Defense (W81XWH-12-10414 BCRP Innovator Expansion), George J. and Angelina P. Kostas Charitable Foundation, Brown Foundation Inc., William Randolph Hearst Foundation, and The Regenerative Medicine Program Cullen Trust for Health Care to E.T.; R.M. was supported by grant RF-2010-2305526; C.C. and A.P. were supported by grant RF-2010-2318372 from Italian Ministry of Health. We thank Associazione Bianca Garavaglia, Via C. Cattaneo, 8, 21052 Busto Arsizio Varese, Italy and Project CREME ‘Campania Research in Experimental Medicine’ POR Campania FSE 2007/2013. We ackowledge D. A. Engler and the Proteomics Core, D. Haviland and the Flow Cytometry Core, A. L. Rivera and the Research Pathology Core at HMRI. We thank M. G. Landry and M. Evangelopoulos for graphical assistance with the creation of the schematics. The authors also acknowledge the Sealy Center for Structural Biology and Molecular Biophysics at the University of Texas Medical Branch at Galveston for providing research resources.
Author information
Authors and Affiliations
Contributions
E.T. conceived the leukosome platform, wrote the paper and was the principal investigator of the major supporting grants. E.T. and R.M. designed the research project and defined the goals of the present study. R.M. developed and optimized the protocols for leukosome assembly, supervised all the experiments, and evaluated the therapeutic efficacy with contributions from J.O.M. and E.D.R.; C.C. performed all the proteomic experiments and the interpretation of the data on protein enrichment; J.O.M. and E.D.R. performed the intravital microscopy experiments and analysis; M.E. performed flow cytometry and optimized the in vitro flow systems. J.O.M. carried out bioluminescence imaging (BLI) analysis and revised the manuscript; F.T. performed FTIR and AFM analyses; S.M. performed DSC analysis; F.T., S.M., and A.D.V. performed H&E and immunofluorescence staining and optical and confocal laser microscopy imaging; M.B.S. performed Cryo-TEM and assisted with analysis; I.K.Y. performed cytokine and organ functionality analyses; P.Z. performed the immunological analysis of leukosomes and gave his expert advice about the study of the immunogenic response; N.E.T.F. performed dexamethasone loading and release experiments; X.W. performed the PCR analysis; A.P. assisted with the editing of the manuscript and mentored the authors during the development of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4419 kb)
Supplementary Information
Supplementary Information (XLSX 40 kb)
Supplementary Information
Supplementary Information (JPG 94 kb)
Rights and permissions
About this article
Cite this article
Molinaro, R., Corbo, C., Martinez, J. et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nature Mater 15, 1037–1046 (2016). https://doi.org/10.1038/nmat4644
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4644
This article is cited by
-
Dendritic cell hybrid nanovaccine for mild heat inspired cancer immunotherapy
Journal of Nanobiotechnology (2023)
-
Engineering M1-derived nanovesicles loading with docosahexaenoic acid synergizes ferroptosis and immune activation for treating hepatocellular carcinoma
Cancer Nanotechnology (2023)
-
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
Nature Reviews Materials (2023)
-
Tyrosine kinase inhibitor-loaded biomimetic nanoparticles as a treatment for osteosarcoma
Cancer Nanotechnology (2022)
-
Targeted neutrophil-mimetic liposomes promote cardiac repair by adsorbing proinflammatory cytokines and regulating the immune microenvironment
Journal of Nanobiotechnology (2022)