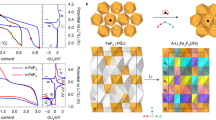
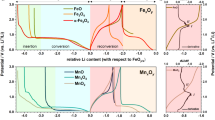
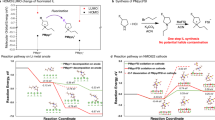
Abstract
Metal fluorides/oxides (MFx/MxOy) are promising electrodes for lithium-ion batteries that operate through conversion reactions. These reactions are associated with much higher energy densities than intercalation reactions. The fluorides/oxides also exhibit additional reversible capacity beyond their theoretical capacity through mechanisms that are still poorly understood, in part owing to the difficulty in characterizing structure at the nanoscale, particularly at buried interfaces. This study employs high-resolution multinuclear/multidimensional solid-state NMR techniques, with in situ synchrotron-based techniques, to study the prototype conversion material RuO2. The experiments, together with theoretical calculations, show that a major contribution to the extra capacity in this system is due to the generation of LiOH and its subsequent reversible reaction with Li to form Li2O and LiH. The research demonstrates a protocol for studying the structure and spatial proximities of nanostructures formed in this system, including the amorphous solid electrolyte interphase that grows on battery electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Idota, Y., Kubota, T., Matsufuji, A., Maekawa, Y. & Miyasaka, T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material. Science 276, 1395–1397 (1997).
Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon, J. M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000).
Amatucci, G. G. & Pereira, N. Fluoride based electrode materials for advanced energy storage devices. J. Fluor. Chem. 128, 243–262 (2007).
Balaya, P., Li, H., Kienle, L. & Maier, J. Fully reversible homogeneous and heterogeneous Li storage in RuO2 with high capacity. Adv. Funct. Mater. 13, 621–625 (2003).
Badway, F., Cosandey, F., Pereira, N. & Amatucci, G. G. Carbon metal fluoride nanocomposites—high-capacity reversible metal fluoride conversion materials as rechargeable positive electrodes for Li batteries. J. Electrochem. Soc. 150, A1318–A1327 (2003).
Li, H., Balaya, P. & Maier, J. Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides. J. Electrochem. Soc. 151, A1878–A1885 (2004).
Li, H., Richter, G. & Maier, J. Reversible formation and decomposition of LiF clusters using transition metal fluorides as precursors and their application in rechargeable Li batteries. Adv. Mater. 15, 736–739 (2003).
Liao, P., MacDonald, B. L., Dunlap, R. A. & Dahn, J. R. Combinatorially prepared [LiF](1-x)Fe-x nanocomposites for positive electrode materials in Li-ion batteries. Chem. Mater. 20, 454–461 (2008).
Cabana, J., Monconduit, L., Larcher, D. & Palacin, M. R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Beaulieu, L. Y., Larcher, D., Dunlap, R. A. & Dahn, J. R. Reaction of Li with grain-boundary atoms in nanostructured compounds. J. Electrochem. Soc. 147, 3206–3212 (2000).
Laruelle, S. et al. On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J. Electrochem. Soc. 149, A627–A634 (2002).
Jamnik, J. & Maier, J. Nanocrystallinity effects in lithium battery materials— Aspects of nano-ionics. Part IV. Phys. Chem. Chem. Phys. 5, 5215–5220 (2003).
Maier, J. Mass storage in space charge regions of nano-sized systems (Nano-ionics. Part V). Faraday Discuss. 134, 51–66 (2007).
Zhukovskii, Y. F., Balaya, P., Kotomin, E. A. & Maier, J. Evidence for interfacial-storage anomaly in nanocomposites for lithium batteries from first-principles simulations. Phys. Rev. Lett. 96, 058302 (2006).
Zhukovskii, Y. F., Balaya, P., Dolle, M., Kotomin, E. A. & Maier, J. Enhanced lithium storage and chemical diffusion in metal-LiF nanocomposites: Experimental and theoretical results. Phys. Rev. B 76, 235414 (2007).
Ponrouch, A., Taberna, P. L., Simon, P. & Palacin, M. R. On the origin of the extra capacity at low potential in materials for Li batteries reacting through conversion reaction. Electrochim. Acta 61, 13–18 (2012).
Menkin, S., Golodnitsky, D. & Peled, E. Artificial solid–electrolyte interphase (SEI) for improved cycleability and safety of lithium-ion cells for EV applications. Electrochem. Commun. 11, 1789–1791 (2009).
Peled, E., Golodnitsky, D., Ulus, A. & Yufit, V. Effect of carbon substrate on SEI composition and morphology. Electrochim. Acta 50, 391–395 (2004).
Eshkenazi, V., Peled, E., Burstein, L. & Golodnitsky, D. XPS analysis of the SEI formed on carbonaceous materials. Solid State Ion. 170, 83–91 (2004).
Ohzuku, T., Sawai, K. & Hirai, T. Topotactic 2-phase reaction of ruthenium dioxide (rutile) in lithium nonaqueous cell. J. Electrochem. Soc. 137, 3004–3010 (1990).
Munoz-Rojas, D., Casas-Cabanas, M. & Baudrin, E. Effect of particle size and cell parameter mismatch on the lithium insertion/deinsertion processes into RuO2 . Solid State Ion. 181, 536–544 (2010).
Bekaert, E., Balaya, P., Murugavel, S., Maier, J. & Menetrier, M. Li-6 MAS NMR investigation of electrochemical lithiation of RuO2: Evidence for an interfacial storage mechanism. Chem. Mater. 21, 856–861 (2009).
Gmitter, A. J. et al. Formation, dynamics, and implication of solid electrolyte interphase in high voltage reversible conversion fluoride nanocomposites. J. Mater. Chem. 20, 4149–4161 (2010).
Leskes, M. et al. Direct detection of discharge products in lithium–oxygen batteries by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. 51, 8560–8563 (2012).
Mackenzie, K.J.D., Smith, & M. E., Multinuclear Solid-State NMR of Inorganic Materials Ch. 6 (Pergamon, 2002).
Ma, Z. R., Zheng, J. P. & Fu, R. Q. Solid state NMR investigation of hydrous ruthenium oxide. Chem. Phys. Lett. 331, 64–70 (2000).
Delmer, O. Size and morphology effects on the cell voltage of Li-batteries: Case Study of RuO 2. PhD thesis, Max Planck Institute, (2009).
Zhuang, G. V., Yang, H., Ross, P. N., Xu, K. & Jow, T. R. Lithium methyl carbonate as a reaction product of metallic lithium and dimethyl carbonate. Electrochem. Solid State 9, A64–A68 (2006).
Borkiewicz, O. J. et al. The AMPIX electrochemical cell: A versatile apparatus for in situ X-ray scattering and spectroscopic measurements. J. Appl. Crystallogr. 45, 1261–1269 (2012).
Chupas, P. J. et al. Rapid-acquisition pair distribution function (RA-PDF) analysis. J. Appl. Crystallogr. 36, 1342–1347 (2003).
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N. & Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 14, 235–248 (1996).
Qui, X., Thompson, J. W. & Billinge, S. J. L. PDFgetX2: A GUI driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystalogr. 37, 678 (2004).
Farrow, C. L. et al. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys. Condens. Mater. 19, 335219 (2007).
Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 43, 1126–1128 (2010).
Giannozzi, P. et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Mater. 21 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Acknowledgements
This research was supported as part of the North Eastern Center for Chemical Energy Storage, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, and Office of Basic Energy Sciences under Award Number DE-SC0001294. Work done at Argonne and use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract No. DE-AC02-06CH11357. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the US DOE, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. Y-Y.H. acknowledges support from a Newton International Fellowship from the Royal Society and a Marie Curie International Incoming Fellowship (PIIF-GA-2011_299341). We thank A. Van der Ven (University of Michigan) and M. Leskes (University of Cambridge) for constructive discussions.
Author information
Authors and Affiliations
Contributions
C.P.G. and Y-Y.H. proposed the concepts and designed the experiments. Y-Y.H., Z.L., K-W.N., O.J.B., X.H., J.C., K.M.W., L-S.D. and X.Y. carried out the experiments, C.P.G., Y-Y.H., K-W.N., O.J.B., X.H., J.C., M.T.D., K.W.C., P.J.C., X.Y. and X-Q.Y. performed the analysis. C.P.G. and Y-Y.H. wrote the manuscript with help from all the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4789 kb)
Rights and permissions
About this article
Cite this article
Hu, YY., Liu, Z., Nam, KW. et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes. Nature Mater 12, 1130–1136 (2013). https://doi.org/10.1038/nmat3784
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3784