Abstract
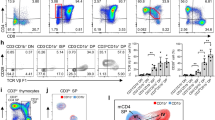
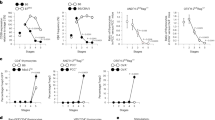
Developing thymocytes are selected for recognition of molecules encoded by the major histocompatibility complex, purged of self-reactive cells and committed to either the CD4 or CD8 lineage. The 1% of thymocytes that complete these tasks emigrate and join the population of peripheral lymphocytes. Whether T cell maturation is complete at the time of thymic exit has been a subject of debate. Using mice transgenic for green fluorescent protein driven by the recombination activating gene 2 promoter to identify recent thymic emigrants, we now show that T cell differentiation continues post-thymically, with progressive maturation of both surface phenotype and immune function. In addition, the relative contribution of CD4 and CD8 recent thymic emigrants was modulated as they entered the peripheral T cell pool. Thus, T cell maturation and subset contribution are both finalized in the lymphoid periphery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kong, F.K., Chen, C.L. & Cooper, M.D. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity 8, 97–104 (1998).
Stutman, O. Intrathymic and extrathymic T cell maturation. Immunol. Rev. 42, 138–184 (1978).
Scollay, R. Thymus cell migration: cells migrating from thymus to peripheral lymphoid organs have a “mature” phenotype. J. Immunol. 128, 1566–1570 (1982).
Scollay, R., Chen, W.F. & Shortman, K. The functional capabilities of cells leaving the thymus. J. Immunol. 132, 25–30 (1984).
Kelly, K.A. & Scollay, R. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int. Immunol. 2, 419–425 (1990).
Gabor, M.J., Godfrey, D.I. & Scollay, R. Recent thymic emigrants are distinct from most medullary thymocytes. Eur. J. Immunol. 27, 2010–2015 (1997).
Tough, D.F. & Sprent, J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179, 1127–1135 (1994).
Berzins, S.P., Boyd, R.L. & Miller, J.F.A.P. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 187, 1839–1848 (1998).
Berzins, S.P., Godfrey, D.I., Miller, J.F.A.P. & Boyd, R.L. A central role for thymic emigrants in peripheral T cell homeostasis. Proc. Natl. Acad. Sci. USA 96, 9787–9791 (1999).
Kong, F.K., Chen, C.L., Six, A., Hockett, R.D. & Cooper, C.J. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc. Natl. Acad. Sci. USA 96, 1536–1540 (1999).
Douek, D.C. et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 396, 690–695 (1998).
Zhang, L. et al. Measuring recent thymic emigrants in blood of normal and HIV-1 infected individuals before and after effective therapy. J. Exp. Med. 190, 725–732 (1999).
Sempowski, G.D., Gooding, M.E., Liao, H.X., Le, P.T. & Haynes, B.F. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol. Immunol. 38, 841–848 (2001).
Ortman, C.L., Dittmar, K.A., Witte, P.L. & Le, P.T. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int. Immunol. 14, 813–822 (2002).
Rodewald, H.R. The thymus in the age of retirement. Nature 396, 630–631 (1998).
Hazenberg, M.D., Verschuren, M.C.M., Hamann, D., Miedema, F. & van Dongen, J.J.M. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. 79, 631–640 (2001).
Hazenberg, M.D., Borghans, J.A.M., de Boer, R.J. & Miedema, F. Thymic output: a bad TREC record. Nat. Immunol. 4, 97–99 (2003).
Kimmig, S. et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 195, 789–794 (2002).
Yu, W. et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature 400, 682–687 (1999).
Nagaoka, H., Gonzalez-Aseguinolaza, G., Tsuji, M. & Nussenzweig, M.C. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J. Exp. Med. 191, 2113–2120 (2000).
Callahan, J.E., Kappler, J.W. & Marrack, P. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 151, 6657–6669 (1993).
McMahan, C.J. & Fink, P.J. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity 9, 637–647 (1998).
Cooper, C.J., Orr, M.T., McMahan, C.J. & Fink, P.J. T cell receptor revision does not solely target recent thymic emigrants. J. Immunol. 171, 226–233 (2003).
Ernst, B., Surh, C.D. & Sprent, J. Thymic selection and cell division. J. Exp. Med. 182, 961–972 (1995).
Penit, C. & Vasseur, F. Expansion of mature thymocyte subsets before emigration to the periphery. J. Immunol. 159, 4848–4856 (1997).
Fink, P.J., Bevan, M.J. & Weissman, I.L. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J. Exp. Med. 159, 436–451 (1984).
Agus, D.B., Surh, C.D. & Sprent, J. Reentry of T cells to the adult thymus is restricted to activated T cells. J. Exp. Med. 173, 1039–1046 (1991).
Gunter, K.C. et al. Thy-1-mediated T-cell activation requires co-expression of CD3/Ti complex. Nature 326, 505–507 (1987).
Feng, C. et al. A potential role for CD69 in thymocyte emigration. Int. Immunol. 14, 535–544 (2002).
Rosen, H., Alfonso, C., Surh, C.D. & McHeyzer-Williams, M.G. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proc. Natl. Acad. Sci. USA 100, 10907–10912 (2003).
Chung, J.B., Silverman, M. & Monroe, J.G. Transitional B cells: step by step towards immune competence. Trends Immunol. 6, 342–348 (2003).
Gartner, F., Alt, F.W., Monroe, R.J. & Seidl, K.J. Antigen-independent appearance of recombination activating gene (RAG)-positive bone marrow B cells in the spleens of immunized mice. J. Exp. Med. 192, 1745–1754 (2000).
Bender, J., Mitchell, T., Kappler, J. & Marrack, P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J. Exp. Med. 190, 367–373 (1999).
Ernst, B., Lee, D.S., Chang, J.M., Sprent, J. & Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Ferreira, C., Barthlott, T., Garcia, S., Zamoyska, R. & Stockinger, B. Differential survival of naive CD4 and CD8 T cells. J. Immunol. 165, 3689–3694 (2000).
Geiselhart, L.A. et al. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J. Immunol. 166, 3019–3027 (2001).
Hassan, J. & Reen, D.J. Human recent thymic emigrants—identification, expansion, and survival characteristics. J. Immunol. 167, 1970–1976 (2001).
Hassan, J. & Reen, D.J. IL-7 and homeostasis of recent thymic emigrants. Trends Immunol. 23, 126–127 (2002).
von Freeden-Jeffry, U., Solvason, N., Howard, M. & Murray, R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity 7, 147–154 (1997).
Tanchot, C. & Rocha, B. Peripheral selection of T cell repertoires: the role of continuous thymic output. J. Exp. Med. 186, 1099–1106 (1997).
Boursalian, T.E. & Bottomly, K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J. Immunol. 162, 3795–3801 (1999).
Dillon, S.R., MacKay, V.L. & Fink, P.J. A functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity 3, 321–333 (1995).
Suzuki, I., Martin, S., Boursalian, T.E., Beers, C. & Fink, P.J. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J. Immunol. 165, 5537–5543 (2000).
Foulds, K.E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002).
Sun, J.C. & Bevan, M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Acknowledgements
Supported by National Institutes of Health grants AG13078 and AI44130 and a pilot grant from the Nathan Shock Center for Excellence in the Basic Biology of Aging (P.J.F.), F32 CA84736 (T.E.B.) and T32 AI07411 (C.J.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Boursalian, T., Golob, J., Soper, D. et al. Continued maturation of thymic emigrants in the periphery. Nat Immunol 5, 418–425 (2004). https://doi.org/10.1038/ni1049
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1049
This article is cited by
-
A guide to thymic selection of T cells
Nature Reviews Immunology (2024)
-
The role of non-canonical Hippo pathway in regulating immune homeostasis
European Journal of Medical Research (2023)
-
PTEN directs developmental and metabolic signaling for innate-like T cell fate and tissue homeostasis
Nature Cell Biology (2022)
-
The impact of the gut microbiota on T cell ontogeny in the thymus
Cellular and Molecular Life Sciences (2022)
-
Essential role of a ThPOK autoregulatory loop in the maintenance of mature CD4+ T cell identity and function
Nature Immunology (2021)