Abstract
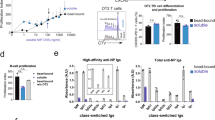
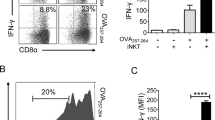
Mouse invariant natural killer T cells (iNKT cells) provide cognate and noncognate help for lipid and protein-specific B cells, respectively. However, the long-term outcome for B cells after cognate help is provided by iNKT cells is unknown at present. Here we found that cognate iNKT cell help resulted in a B cell differentiation program characterized by extrafollicular plasmablasts, germinal-center formation, affinity maturation and a robust primary immunoglobulin G (IgG) antibody response that was uniquely dependent on iNKT cell–derived interleukin 21 (IL-21). However, cognate help from iNKT cells did not generate an enhanced humoral memory response. Thus, cognate iNKT cell help for lipid-specific B cells induces a unique signature that is a hybrid of classic T cell–dependent and T cell–independent type 2 B cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
MacLennan, I.C. et al. Extrafollicular antibody responses. Immunol. Rev. 194, 8–18 (2003).
Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392 (1991).
Leadbetter, E.A. et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. USA 105, 8339–8344 (2008).
Barral, P. et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. USA 105, 8345–8350 (2008).
Godfrey, D.I., MacDonald, H.R., Kronenberg, M., Smyth, M.J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Roark, J.H. et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 160, 3121–3127 (1998).
Mandal, M. et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol. Immunol. 35, 525–536 (1998).
Cohen, N.R., Garg, S. & Brenner, M.B. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv. Immunol. 102, 1–94 (2009).
Bendelac, A., Matzinger, P., Seder, R.A., Paul, W.E. & Schwartz, R.H. Activation events during thymic selection. J. Exp. Med. 175, 731–742 (1992).
Uldrich, A.P. et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 175, 3092–3101 (2005).
Kumar, H., Belperron, A., Barthold, S.W. & Bockenstedt, L.K. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165, 4797–4801 (2000).
Belperron, A.A., Dailey, C.M. & Bockenstedt, L.K. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174, 5681–5686 (2005).
Kobrynski, L.J., Sousa, A.O., Nahmias, A.J. & Lee, F.K. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J. Immunol. 174, 1787–1790 (2005).
Schofield, L. et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283, 225–229 (1999).
Bialecki, E. et al. Role of marginal zone B lymphocytes in invariant NKT cell activation. J. Immunol. 182, 6105–6113 (2009).
Muppidi, J.R. et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J. Exp. Med. 208, 1941–1948 (2011).
Galli, G. et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA 104, 3984–3989 (2007).
Devera, T.S., Shah, H.B., Lang, G.A. & Lang, M.L. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur. J. Immunol. 38, 1001–1011 (2008).
MacLennan, I.C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Herzenberg, L.A., Black, S.J. & Tokuhisa, T. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J. Exp. Med. 151, 1071–1087 (1980).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Fazilleau, N., Mark, L., McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Follicular helper T cells: lineage and location. Immunity 30, 324–335 (2009).
Johnston, B., Kim, C.H., Soler, D., Emoto, M. & Butcher, E.C. Differential chemokine responses and homing patterns of murine TCRαβ NKT cell subsets. J. Immunol. 171, 2960–2969 (2003).
Coquet, J.M. et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 178, 2827–2834 (2007).
King, I.L., Mohrs, K. & Mohrs, M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J. Immunol. 185, 6138–6145 (2010).
Linterman, M.A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Vinuesa, C.G., Tangye, S.G., Moser, B. & Mackay, C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5, 853–865 (2005).
Hayakawa, Y. et al. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166, 6012–6018 (2001).
Ridderstad, A. & Tarlinton, D.M. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J. Immunol. 160, 4688–4695 (1998).
Randall, K.L. et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat. Immunol. 10, 1283–1291 (2009).
Vinuesa, C.G., Linterman, M.A., Goodnow, C.C. & Randall, K.L. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 237, 72–89 (2010).
Good-Jacobson, K.L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542 (2010).
Anderson, S.M., Tomayko, M.M., Ahuja, A., Haberman, A.M. & Shlomchik, M.J. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 204, 2103–2114 (2007).
Stetson, D.B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Muppidi, J.R. et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J. Exp. Med. 208, 1941–1948 (2011).
Cyster, J.G. B cells on the front line. Nat. Immunol. 1, 9–10 (2000).
Lopes-Carvalho, T., Foote, J. & Kearney, J.F. Marginal zone B cells in lymphocyte activation and regulation. Curr. Opin. Immunol. 17, 244–250 (2005).
Shih, T.-A.Y., Roederer, M. & Nussenzweig, M.C. Role of antigen-receptor affinity in T cell-independent antibody responses in vivo. Nat. Immunol. 3, 399–406 (2002).
Bendelac, A., Hunziker, R.D. & Lantz, O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184, 1285–1293 (1996).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 28, 1623–1626 (1997).
Lam, KP., Kuhn, R. & Rajewsky, K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90, 1073–1083 (1997).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002).
Eaton, S.M., Burns, E.M., Kusser, K., Randall, T.D. & Haynes, L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J. Exp. Med. 200, 1613–1622 (2004).
Acknowledgements
We thank M. Nussenzweig (Rockefeller University) for B6.SJL B1-8hi mice; M. Exley (Dana Farber Cancer Institute) for C57BL/6 Vα14-transgenic mice and C57BL/6 Jα18-deficient mice; K. Rajewsky (Center for Blood Research) for B1-8f mice; M. Rincon (University of Vermont) for IL-21-deficient mice; and the US National Institutes of Health Tetramer Core for mouse CD1d-PBS57 tetramers and unloaded CD1d tetramers. Supported by the Trudeau Institute (E.A.L.), the US National Institutes of Health (AI028973-23 and AI063428-06 to M.B.B. and T32 A1049823-10 to I.L.K.), J. Bardrick (G.S.B.), the Royal Society (G.S.B.), The Wellcome Trust (084923/B/08/Z to G.S.B.) and the Medical Research Council (G.S.B.).
Author information
Authors and Affiliations
Contributions
I.L.K. designed and did experiments, analyzed data and edited the manuscript; A.F., M.T., J.D. and G.F.M.W. designed and did experiments; A.M.H. did experiments, edited the manuscript and provided technical advice; N.V. and G.S.B. synthesized and provided lipid antigens; M.M. and M.B.B. provided conceptual advice and E.A.L. initiated and directed the research, did experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 1298 kb)
Rights and permissions
About this article
Cite this article
King, I., Fortier, A., Tighe, M. et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol 13, 44–50 (2012). https://doi.org/10.1038/ni.2172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2172
This article is cited by
-
Zeb2 regulates differentiation of long-lived effector of invariant natural killer T cells
Communications Biology (2023)
-
Developmentally programmed early-age skin localization of iNKT cells supports local tissue development and homeostasis
Nature Immunology (2023)
-
Re-programming mouse liver-resident invariant natural killer T cells for suppressing hepatic and diabetogenic autoimmunity
Nature Communications (2022)
-
The role of invariant T cells in inflammation of the skin and airways
Seminars in Immunopathology (2019)
-
Tissue-specific functions of invariant natural killer T cells
Nature Reviews Immunology (2018)