Abstract
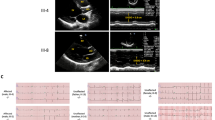
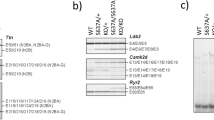
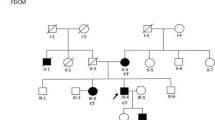
Stress tolerance of the heart requires high-fidelity metabolic sensing by ATP-sensitive potassium (KATP) channels that adjust membrane potential–dependent functions to match cellular energetic demand. Scanning of genomic DNA from individuals with heart failure and rhythm disturbances due to idiopathic dilated cardiomyopathy identified two mutations in ABCC9, which encodes the regulatory SUR2A subunit of the cardiac KATP channel. These missense and frameshift mutations mapped to evolutionarily conserved domains adjacent to the catalytic ATPase pocket within SUR2A. Mutant SUR2A proteins showed aberrant redistribution of conformations in the intrinsic ATP hydrolytic cycle, translating into abnormal KATP channel phenotypes with compromised metabolic signal decoding. Defective catalysis-mediated pore regulation is thus a mechanism for channel dysfunction and susceptibility to dilated cardiomyopathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Inagaki, N. et al. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 270, 1166– 1170 (1995).
Inagaki, N. et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16, 1011– 1017 (1996).
Bienengraeber, M. et al. ATPase activity of the sulfonylurea receptor: A catalytic function for the KATP channel complex. FASEB J. 14, 1943– 1952 (2000).
Matsuo, M., Tanabe, K., Kioka, N., Amachi, T. & Ueda, K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J. Biol. Chem. 275, 28757– 28763 (2000).
Zingman, L.V. et al. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron 31, 233– 245 (2001).
Tucker, S.J., Gribble, F., Zhao, C., Trapp, S. & Ashcroft, F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387, 179– 183 (1997).
Zingman, L.V. et al. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J. Biol. Chem. 277, 14206– 14210 (2002).
Weiss, J.N. & Lamp, S.T. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science 238, 67– 69 (1987).
O'Rourke, B., Ramza, B. & Marban, E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265, 962– 966 (1994).
Nichols, C.G. et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272, 1785– 1787 (1996).
Yamada, K. et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science 292, 1543– 1546 (2001).
Zingman, L.V. et al. Kir6.2 is required for adaptation to stress. Proc. Natl. Acad. Sci. USA 99, 13278– 13283 (2002).
Hodgson, D.M. et al. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 22, 1532– 1542 (2003).
Schmitt, J.P. et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299, 1410– 1413 (2003).
Chien, K.R., Ross, J. & Hoshijima, M. Calcium and heart failure. Nat. Med. 9, 508– 509 (2003).
Chien, K.R. Stress pathways and heart failure. Cell 98, 555– 558 (1999).
Seidman, J.G. & Seidman, C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell 104, 557– 567 (2001).
Towbin, J.A. & Bowles, N.E. The failing heart. Nature 415, 227– 233 (2002).
Sharma, N. et al. The C terminus of SUR1 is required for trafficking of KATP channels. J. Biol. Chem. 274, 20628– 20632 (1999).
Cartier, E.A., Conti, L.R., Vandenberg, C.A. & Shyng, S.L. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc. Natl. Acad. Sci. USA 98, 2882– 2887 (2001).
Walker, J.E., Saraste, M., Runswick, M. & Gay, N. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945– 951 (1982).
Matsushita, K. et al. Intramolecular interaction of SUR2 subtypes for intracellular ADP-induced differential control of KATP channels. Circ. Res. 90, 554– 561 (2002).
Abraham, M.R. et al. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem. 277, 24427– 24434 (2002).
Noma, A. ATP-regulated K+ channels in cardiac muscle. Nature 305, 147– 148 (1983).
Nestorowicz, A. et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes 46, 1743– 1748 (1997).
Olson, T.M. et al. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science 280, 750– 752 (1998).
Gaudet, R. & Wiley, D.C. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 20, 4964– 4972 (2001).
Pang, Y.P. Successful molecular dynamics simulation of two zinc complexes bridged by a hydroxide in phosphotriesterase using the cationic dummy atom method. Proteins 45, 183– 189 (2001).
Schwappach, B., Zerangue, N., Jan, Y.N. & Jan, L.Y. Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron 26, 155– 167 (2000).
Acknowledgements
We thank J. Bryan, Y. Kurachi and S. Seino for KATP channel clones; B. Schwappach for constructs used in trafficking studies; and T.P. Burghardt and A.J. Caride for technical advice. This work was supported by the US National Institutes of Health, American Heart Association, Miami Heart Research Institute, Marriott Foundation, Siragusa Foundation, University of Minnesota Supercomputing Institute, Mayo-Dubai Healthcare City Research Project and Mayo Foundation. A.T. is an Established Investigator of the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bienengraeber, M., Olson, T., Selivanov, V. et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet 36, 382–387 (2004). https://doi.org/10.1038/ng1329
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1329