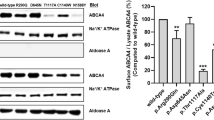
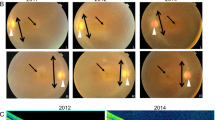
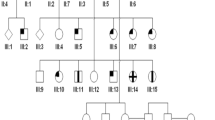
Abstract
Stargardt disease (STGD, also known as fundus flavimaculatus; FFM) is an autosomal recessive retinal disorder characterized by a juvenile-onset macular dystrophy, alterations of the peripheral retina, and subretinal deposition of lipofuscin-like material. A gene encoding an ATP-binding cassette (ABC) transporter was mapped to the 2-cM (centiMorgan) interval at 1p13-p21 previously shown by linkage analysis to harbour the STGD gene. This gene, ABCR, is expressed exclusively and at high levels in the retina, in rod but not cone photoreceptors, as detected by in situ hybridization. Mutational analysis of ABCR in STGD families revealed a total of 19 different mutations including homozygous mutations in two families with consanguineous parentage. These data indicate that ABCR is the causal gene of STGD/FFM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
National Advisory Eye Council. Vision Research, a National Plan, 1994-1998. National Institutes of Health publication 93–3186 (1993).
Blacharski, P.A. Fundus flavimaculatus. in Retinal dystrophies and degenerations,(ed. Newsome, D.A.) 135–159 (New York: Raven Press, 1988).
Stargardt, K. U¨ber fämiliare, progressive degeneration in der maculagegend des auges. Albrechtvon Graefes. Arch Klin. Exp. Ophthalmol. 71, 534–550 (1909).
Anderson, K.L. et al. A YAC contig encompassing the recessive Stargardt disease gene (STGD) on chromosome 1p. Am. J. Hum. Genet. 57, 1351–1363 (1995).
Franceschetti, A. Über tapeto-retinale degenerationen im kindesalter. in Entwicklung und fortschritt in der augenheilkunde. (ed von Sautter, H.) 107–120 (Stuttgart: Ferdinand Enke, 1963).
Fishman, G.A. Fundus flavimaculatus: a clinical classification. Arch. Ophthalmol. 94, 2061–2067 (1976).
Noble, K.G. & Carr, R.E. Stargardt's disease and fundus flavimaculatus. Arch. Ophthalmol. 97, 1281–1285 (1979).
Kaplan, J. et al. A gene for Stargardt's disease (fundus flavimaculatus) maps to the short arm of chromosome 1. Nature Genet. 5, 308–311 (1993).
Gerber, S. et al. A gene for late-onset fundus flavimaculatus with macular dystrophy maps to chromosome 1p13. Am. J. Hum. Genet. 56, 396–399 (1995).
Hoyng, C.B. et al. Genetic fine mapping of the gene for recessive Stargardt disease. Hum. Genet. 98, 500–504 (1996).
Zhang, K. et al. A dominant Stargardt's macular dystrophy locus maps to chromosome 13q34. Arch. Ophthalmol. 112, 759–764 (1994).
Stone, E.M. et al. Clinical features of a Stargardt-like dominant progressive macular dystrophy with genetic linkage to chromosome 6q. Arch. Ophthalmol. 112, 765–772 (1994).
Gass, J.D.M. Stargardt's disease (Fundus Flavimaculatus). in Stereoscopic atlas of macular diseases: diagnosis and treatment (ed. Gass, J.D.M.) 256–261 (St Louis, Missouri: C.V. Mosby, 1987).
Childs, S. & Ling, V. The MDR super-family of genes and its biological implications, in Important advances in oncology (eds V. T. DeVita, S. Hellman, S. & Rosenberg, S.A.) 21–36 (Philadelphia, Pennsylvania: Lippincott Company, 1994).
Dean, M. & Allikmets, R. Evolution of ATP-binding cassette transporter genes. Curr. Opin. Genet. Dev. 5, 779–785 (1995).
Riordan, J.R. et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073 (1989).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Thomas, P.M. et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 268, 426–429 (1995).
Shimozawa, N. et al. A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science 255, 1132–1134 (1992).
de la Salle, H. et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science 265, 237–241 (1994).
Hyde, S.C. et al. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346, 362–365 (1990).
Michaelis, S. & Berkower, C. Sequence comparison of yeast ATP binding casette (ABC) proteins, in Cold Spring Harbor Symposium on Quantitative Biology, vol. LX: Protein kinesis - the dynamics of protein trafficking and stability. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1995).
Allikmets, R. Gerrard, B. Court, D. & Dean, M. Cloning and organization of the abc and mdl genes of Escherichia coli: relationship to eukaryotic multidrug resistance. Gene 136, 231–236 (1993).
Allikmets, R. et al. Characterization and mapping of three new mammalian ATP-binding transporter genes from an EST database. Mamm. Genome 6, 114–117 (1995).
Dean, M. et al. Mapping and sequencing of two yeast genes belonging to the ATP-binding cassette superfamily. Yeast 10, 377–383 (1994).
Luciani, M.-F. Denizot, F. Savary, S. Mattel, M.G. & Chimini, G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 21, 150–159 (1994).
Allikmets, R. Gerrard, B. Hutchinson, A. & Dean, M. Characterization of the human ABC superfamily: Isolation and mapping of 21 new genes using the expressed sequence tags database. Hum. Mol. Genet. 5, 1649–1655 (1996).
Lennon, G. Auffray, C. Polymeropoulos, M. & Scares, M.B. The I. M.A.G.E. consortium: An integrated molecular analysis of genomes and their expression. Genomics 33, 151–152 (1996).
Klugbauer, N. & Hofmann, F. Primary structure of a novel ABC transporter with a chromosomal localization on the band encoding the multidrug resistance-associated protein. FEBS Lett. 391, 61–65 (1996).
Anderson, K.L. Towards the isolation of genes recessively inherited ocular disorders. Ph.D. thesis. Baylor College of Medicine (1996).
Luciani, M.-F. & Chimini, G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 15, 226–235 (1996).
Zinn, K.M. & Marmor, M.F. The retinal pigment epithelium. 521 (Harvard University Press, Cambridge. MA, 1979).
Dowling, J.E. Chemistry of visual adaptation in the rat. Nature 188, 114–118 (1960).
Anderson, R.E. & Maude, M.B. Lipids of ocular tissues: the effects of essential fatty acid deficiency on the phospholipids of the photoreceptor membranes of rat retina. Arch. Biochem. Biophys. 151, 270–276 (1971).
Rando, R.R. The chemistry of vitamin A and vision. Angew. Chem. Int. Ed. Engl. 29, 461–480 (1990).
Fong, S.-L. Liou, G.I. Landers, R.A. Alvarez, R.A. & CD.Purification and characterization of a retinol-binding glycoprotein synthesized and secreted by bovine neural retina. J. Biol. Chem. 259, 6534–6542 (1984).
Daemen, F.J.M. Vertebrate rod outer segment membranes. Biochem. Biophys. Acta 300. 255–288 (1973).
Hayes, K.C. Retinal degeneration in monkeys induced by deficiencies of vitamin E or A. Invest. Ophthalmol. 13, 499–510, (1974).
Hettema, E.H. et al. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 15, 3813–3822 (1996).
Shani, N. & Valle, D. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters.Proc. Natl. Acad. Sci. USA 93, 11901–11906 (1996).
Smit, J.J.M. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
van Helvoort, A. et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87, 507–517 (1996).
Klein, B.A. & Krill, A.E. Flavimaculatus: clinical, functional, and histopathologic observations. Am. J. Ophthalmol. 64, 3–23 (1967).
Eagle, R.C. Jr. Lucier, A.C. Bernadino, V.B. & Yanoff, M. Retinal pigment epithelial abnormalities in Fundus Flavimaculatus: A light and electron microscopic study. Ophthalmology 87, 1189–1200 (1980).
McDonnell, P.J. Kivlin, J.D. Maumenee, I.H. & Green, W.R. Fundus flavimaculatus without maculopathy: a clinicopathologic study. Ophthalmology 93, 116–119 (1986).
Lopez, P.F. Maumenee, I.H. de la Cruz, Z. & Green, W.R. Autosomal-dominant fundus flavimaculatus: clinicopathologic correlation. Ophthalmology 97, 798–809 (1990).
Steinmetz, R.L. Garner, A. Maguire, J.I. & Bird, A.C. Histopathology of incipient fundus flavimaculatus. Ophthalmology 98, 953–956 (1991).
Birnbach, C.D. Järveläinen, M. Possin, D.E. & Milam, A.H. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology 101, 1211–1219 (1994).
Wing, G.L. Blanchard, G.C. & Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 17, 601–607 (1978).
Weiter, J.J. Delori, F.C. Wing, G.L. & Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest. Ophthalmol. Vis. Sci. 27, 145–152 (1986).
Dryja, T.P. & Li, T. Molecular genetics of retinitis pigmentosa. Hum. Mol. Genet. 4, 1739–1743 (1995).
Meindl, A. et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nature Genet. 13, 35–42 (1996).
Seabra, M.C. Brown, M.S. & Goldstein, J.L. Retinal degeneration in choroideremia: deficiency of Rab geranylgeranyl transferase. Science 259, 377–381 (1993).
Valle, D. & Simell, O. The hyperornithinemias. in The metabolic and molecular basis of inherited disease (eds Scriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 1147–1185 (McGraw Hill, New York, 1995).
Weber, B.H.F. Vogt, G. Pruett, R.C. Stohr, H. & Felbor, U. Mutations in the tissue inhibitor of met alloproteinase-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nature Genet. 8, 352–355 (1994).
Meindl, A. et al. Norrie disease is caused by mutations in an extracellular protein resembling the c-terminal globular domain of mucins. Nature Genet. 2, 139–143 (1992).
Meitinger, T. et al. Molecular modelling of the Norrie disease protein predicts a cystihe knot growth factor tertiary structure. Nature Genet. 5, 376–380 (1993).
Boguski, M.S. Lowe, T.M. & Tolstoshev, C.M. stoshev, C.M. dbEST-database for ‘expressed sequence tags’. Nature Genet. 4, 332–333 (1993).
Altschul, S.F. Gish, W. Miller, W. Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Feng, D.-F. & Doolittle, R.F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J. Mol. Evol. 25, 351–360 (1987).
Devereaux, J. Haeberli, P. & Smithies, O. A comprehensive set of sequence analysis programs for the VAX. Nucl. Acids Res. 12, 387–395 (1984).
Nathans, J. Thomas, D. & Hogness, D.S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science 232, 193–202 (1986).
Lincoln, A.L. Daly, M. & Lander, E. PRIMER: a computer program for automatically selecting PCR primers. Whitehead Institute Technical Report (1991).
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Sambrook, J. Fritsch, E.F. & Maniatis, T. Molecular Cloning. (Cold Spring Harbor Laboratory press, New York, 1989).
Orita, M. Suzuki, Y. Sekiya, T. & Hayashi, K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 5, 874–879 (1989).
White, M. Carvalho, M. Derse, D. O'Brien, S.J. & Dean, M. Detecting single base substitutions as heteroduplex polymorphisms. Genomics 12, 301–306 (1992).
Glavač, D. & Dean, M. Optimization of the single strand conformation polymorphism (SSCP) technique for detection of point mutations. Hum. Mutat. 2, 404–414 (1993).
Roa, B.B. et al. Charcot-Marie-Tooth disease type 1A, association with a spontaneous point mutation in the PMP22 gene. New Engl. J. Med. 329, 96–101 (1993).
Roa, B.B. et al. Myelin protein zero (MPZ) gene mutations in nonduplication type 1 Charcot- Marie-Tooth disease. Hum. Mut. 7, 36–45 (1996).
Warner, L.E. et al. Clinical phenotypes of different MPZ(P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas, and congenital hypomyelination. Neuron 17, 451–460 (1996).
Rowe, L.B. et al. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm. Genome 5, 253–274 (1994).
Schaeren-Wiemers, N. & Gerfin-Moser, A. A single protocol to detect transcripts of various types and expression levels in neural tissue and culture cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993).
Zhou, H. Yoshioka, T. & Nathans, J. POU-domain factor-1: a complex POU-domain gene implicated in the development of retinal ganglion and amacrine cells. J. Neurosci. 16, 2261–2274 (1996).
Chiu, M.I. Zack, D.J. Wang, Y. & Nathans, J. Murine and bovine blue cone pigment genes: cloning and characterization of two new members of the S family of visual pigments. Genomics 21, 440–443 (1994).
Bellanne-Chantelot, C. et al. Mapping the whole human genome by fingerprinting yeast artificial chromosomes. Cell 70, 1059–1068 (1992).
Kuwano, Y. Nakanishi, O. Nabeshima, Y.-I. Tanaka, T. & Ogata, K. Molecular cloning and nucleotide sequence of DNA complementary to rat ribosomal protein S26 messenger RNA. J. Biochem. (Tokyo) 97, 983–992 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allikmets, R., Singh, N., Sun, H. et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Starqardt macular dystrophy. Nat Genet 15, 236–246 (1997). https://doi.org/10.1038/ng0397-236
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0397-236