Abstract
The structurally complex alkaloid gelsemine was previously thought to have no significant biological activities, but a recent study has shown that it has potent and specific antinociception in chronic pain. While this molecule has attracted significant interests from the synthetic community, an efficient synthetic strategy is still the goal of many synthetic chemists. Here we report the asymmetric total synthesis of (+)-gelsemine, including a highly diastereoselective and enantioselective organocatalytic Diels–Alder reaction, an efficient intramolecular trans-annular aldol condensation furnishing the prolidine ring and establishing the configuration of the C20 quaternary carbon stereochemical centre. The entire gelsemine skeleton was constructed through a late-stage intramolecular SN2 substitution. The enantiomeric excess of this total synthesis is over 99%, and the overall yield is around 5%.
Similar content being viewed by others
Introduction
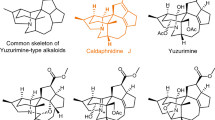
Although gelsemine was isolated1 in as early as 1876 from Gelsemium Sempervirens Ait., its structure was not determined until 1959 by means of nuclear magnetic resonance (NMR) spectroscopic techniques2,3 and X-ray crystallographic analysis4. This indole alkaloid contains a hexacyclic cage structure and seven contiguous chiral carbon centres (Fig. 1). The complex chemical structures of gelsemine and other members of the alkaloid family5,6,7,8 have attracted considerable attention from synthetic chemists. So far, in addition to the many synthetic efforts9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37, there are eight total syntheses reported in the literature38,39,40,41,42,43,44,45,46,47,48,49 (Fig. 2), two of which are asymmetric44,48. Although gelsemine was thought to have no particular biological activities, a recent report indicated that gelsemine exhibited potent and specific antinociception in chronic pain by acting at the three spinal glycine receptors50. Besides, gelsemine was nonaddictive, indicating that the mechanism of its action is different from that of morphine. The complex structure and the potential medicinal applications of gelsemine prompted us to initiate a more efficient enantioselective total synthesis.
Herein we wish to report a 12-step, highly enantioselective organocatalytic total synthesis of (+)-gelsemine.
Results
Retrosynthetic analysis
Gelsemine may be synthesized from intermediate RS-1 and oxindole via the condensation of the hemiacetal with oxindole followed by an intramolecular SN2 displacement (Fig. 3). Although the condensation may result in four stereoisomers, only two of them may undergo the desired SN2 displacement. The other two isomers, however, may either stay intact or undergo an elimination followed by a Michael addition51,52 to regenerate the four stereoisomers. This equilibrium is shifted to form the desired product after the intramolecular SN2 displacement, which is irreversible under the reaction conditions (Figs 4 and 5). The SN2 displacement may result in two isomers, one of which is the desired product. Intermediate RS-1 may be obtained from RS-2 following a sequence of intramolecular aldol condensation, reduction of the carbonyl group, formation of the sulfonates and then elimination. The intramolecular aldol53,54 condensation deserves further discussion due to the fact that both the aldehyde and the ketone functionalities may undergo enolization under the reaction conditions, resulting in epimerization of both stereochemical centres attaching the carbonyl groups. Another issue is the direction of the aldol condensation. Since both of the carbonyl groups may be enolized, the aldol condensation from either one may be consequential. However, Cbz is a bulky functional group55 and it will play a significant role in preventing the aldehyde from being enolized prior to the ketone enolization. In this case, the potential epimerization of the ketone functionality is irrelevant. The third issue is the stereochemistry of the hydroxyl group even if aldol condensation occurs in the desired direction. This difficulty may be overcome when one realizes that the desired product has a more favourable internal hydrogen bond56,57 than the other isomer. Finally, formation of RS-3 and its conversion into RS-2 is straightforward.
In principle, gelsemine may be constructed from oxindole and intermediate RS-1, where X is a leaving group. After a few transformations, RS-1 may be synthesized from intermediate RS-2, which inturn may be obtained from RS-3 following several reaction steps including ozonolysis. Finally, RS-3 may be synthesized from readily accessible starting materials.
Synthesis of the (+)-gelsemine
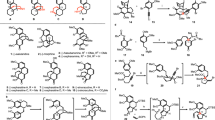
On the basis of the above analysis, the synthetic strategy seemed feasible. If intermediate 3 is made asymmetric, then gelsemine will be made asymmetric. Thus, after a brief literature search58,59, an asymmetric Diels–Alder reaction was designed and the synthesis began with dihydropyridine 1 (Fig. 6), which may be prepared from 4-methylpyridine in large scale60.
Reagents and conditions: (a) Cat. (0.1 eq), CH3CN/H2O (20:1), −20 °C, 36 h, then NaBH4 (1 eq), 0 °C, 30% for 3a, 47% for 3; (b) DBU, toluene, reflux, 20 h, 97%; (c) Dibal-H (1.05 eq), DCM, −78 °C, 3 h, 90%; (d) KHMDS (4.4 eq), MOMPPh3Cl (4 eq), THF, 0 °C—rt, 3 h, then 4, 0 °C, 4 h; pTSA (0.1 eq), CH(OMe)3, DCM, rt, 93% for 5a and 5 (5a:5=1:13); (e) pTSA (0.1 eq), CH(OMe)3, DCM, rt; (f) O3, DCM, −78 °C, 30 min; NaOCH3 (0.3 eq), CH3OH, 0 °C, 24 h, 60%; (g) NaBH4 (1.1 eq), CH3OH, 0 °C, 30 min, 93%; (h) MsCl (3 eq), DMAP (3 eq), Et3N (5 eq), DCM, 0 °C, quantitative; (i) DBU, toluene, reflux, 24 h, 85%; (j) LiAlH4 (1.2 eq), THF, 0 °C, 10 h, 86%; (k) 6 M HCl, THF, H2O, 3 h, 96%; (l) piperidine, 1-MOM-oxindole (1.5 eq), CH3OH, reflux, 86%; (m) LDA (1.2 eq), Et2AlCl (5 eq), toluene, 32%; (n) 6 M HCl, THF, 50 °C, 24 h; Et3N, CH3OH, 55 °C, 24 h, 70%. DBU, 1,8-diazabicycloundec-7-ene; Dibal-H, diisobutyl aluminium hydride; KHMDS, potassium hexamethyldisilazane; pTSA, p-toluenesulfonic acid; DCM, dichloromethane; MsCl, methanesulfonyl chloride; DMAP, 4-dimethylaminopyridine; LDA, lithium diisopropylamide; rt, room temperature.
Gratifyingly, the yield of the desired endo product was 47% after reduction of the aldehyde carbonyl group with sodium borohydride, and its enantio excess was determined using chiral high-performance liquid chromatography (HPLC) to be 99.7%, while the exo product was not detected. It was surprising that intermediate 3a was also produced in 30% yield. Since intermediate 3 was stable under the reaction conditions, 3a may be a result of the double-bond isomerization of the enal during the catalytic process61, and the rate of the double-bond isomerization was comparable to that of the Diels–Alder cycloaddition (Fig. 7). Fortunately, 3a was converted into 3 with DBU (1,8-diazabicycloundec-7-ene) in refluxing toluene in 97% yield, which brought the total yield of the Diels–Alder cycloaddition to 76%. Intermediate 3 was then further selectively reduced to the hemiacetal 4 using Dibal-H at −78 °C in 94% yield. The subsequent Wittig reaction furnished the methyl enol ether, which was directly treated with trimethyl orthoformate and a catalytic amount of p-toluenesulfonic acid to provide intermediate 5 and 5a (13:1) as a separable mixture in 93% combined yield. Although 5a may be used as well, it was converted into 5 by treating it with pTSOH in methylene chloride (DCM) and only 5 was used for the next step. After a conventional ozonolysis of intermediate 5 in DCM, the resulting dicarbonyl intermediate was directly treated with sodium methoxide in methanol at 0 °C due to the fact that the dicarbonyl intermediate was unstable for storage. To our delight, the aldol reaction afforded the desired product 6 in 60% combined yield. However, the reaction of 6 with the methanesulfonyl chloride resulted in a complex mixture. Thus, the hydroxyketone intermediate 6 was reduced to diol 7 with sodium borohydride (97%) and the formation of disulfonate 8 with methanesulfonyl chloride was quantitative, the structure of which was confirmed through X-ray crystallographic analysis (Fig. 8). Treatment of intermediate 8 with DBU (1,8-diazabicycloundec-7-ene) in refluxing toluene led to the formation of alkene 9 (85%) and reduction of the Cbz protective group to methyl with lithium aluminium hydride in THF afforded 10 in 86% yield. Subsequent acid hydrolysis of the acetal with aqueous hydrochloric acid in THF provided hemiacetal 11 (96%).
With the key intermediate in hand, we began to test the condensation of 11 with methoxymethyl oxindole and the subsequent SN2 displacement, another key reaction for the synthesis of gelsemine. As expected, the condensation of intermediate 11 with oxindole in refluxing methanol and a catalytic amount of piperidine afforded the desired product 12 (85%) as an inseparable mixture of all four possible isomers. The seemed straightforward intramolecular SN2 substitution reaction turned out to be problematic. Many reaction conditions were tested (NaH/THF; NaOCH3/CH3OH; KOtBu/THF; KOtBu/THF/ButOH; LDA/THF; CsF/DMF62; LiHMDS/THF; LiHMDS/HMPA/THF; LiHMDS/LiCl/THF, LiHMDS/ZnCl2/THF; LiHMDS/DMSO; LDA/Et2AlCl/THF; LiHMDS/Me2AlCl/toluene; LiHMDS/Me2AlCl/THF; NaHMDS/Me2AlCl/THF, NaH/DMF) but all turned into a complex product mixture. However, when intermediate 12 was treated with LDA and then diethylaluminum chloride in toluene at 90 °C, the reaction furnished the desired product in 32% yield as a single isomer. Finally, acid hydrolysis of the methyl group from the methoxymethyl protective group and removal of the resulting hydroxymethyl with triethylamine converted 13 into (+)-gelsemine in 70% combined yield. The synthetic material is identical to the natural product in terms of carbon and proton NMR spectra and optical rotation (see Supplementary Fig. 15).
Discussion
The total synthesis of (+)-gelsemine is completed in a highly enantioselective manner from readily accessible starting materials. This synthesis features an enantioselective organocatalytic Diels–Alder reaction, a formidable intramolecular aldol cyclization and a challenging intramolecular SN2 displacement. The combination of all these features resulted in exceptional overall synthetic efficiency: the enantio excess is over 99%, and the total yield is about 5%.
Methods
General
All reagents were reagent grade and used without purification, unless otherwise noted. All reactions involving air- or moisture-sensitive reagents or intermediates were performed under an inert atmosphere of argon in glassware that was oven dried. Reaction temperatures referred to the temperature of the cooling/heating bath. Chromatography was performed using forced flow (flash chromatography) of the indicated solvent system on 230-400 mesh silica gel (Silicycle flash F60), unless otherwise noted. 1H NMR and 13C NMR spectra were recorded on a Bruker AV-400 or 500 MHz spectrometer. Chemical shifts were referenced to the deuterated solvent (for example, for CDCl3, δ=7.27 p.p.m. and 77.0 p.p.m. for 1H and 13C NMR, respectively) and reported in parts per million (p.p.m., δ) relative to tetramethylsilane (δ=0.00 p.p.m.). Coupling constants (J) were reported in Hz and the splitting abbreviations used were: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; comp, overlapping multiplets of magnetically non-equivalent protons; br, broad; app, apparent. Reactions were monitored using thin-layer chromatography carried out on 0.25-mm E. Merck silica gel plates (60F-254) using ultraviolet light as the visualizing agent or an ethanolic solution of phosphomolybdic acid, cerium sulfate and heat as developing agents. Optical rotations were measured on a PerkinElmer 341 polarimeter. Enantiomeric ratios were determined by chiral HPLC using a chiralpak AD-H (amylose tris(3,5-dimethylphenylcarbamate) coated on 5-μm silica gel) with hexane and i-PrOH as eluents. Tetrahydrofuran, benzene, toluene and diethyl ether were distilled from Na and diphenylketone. DCM, N,N-diisopropylethylamine and triethylamine were distilled from calcium hydride, while methanol was distilled from dry magnesium turnings immediately before use.
For 1H and 13C NMR spectra of compounds, see Supplementary Figs 1–14. For the comparisons of 1H spectra of the natural and synthetic gelsemine, see Supplementary Fig. 15. For the HPLC of 3, see Supplementary Fig. 16. For the experimental procedures and spectroscopic and physical data of compounds and the crystallographic data of compound 8, see Supplementary Methods.
Additional information
Accession codes: The X-ray crystallographic coordinates for structures 8 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1056043. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Chen, X. et al. Total synthesis of (+)-gelsemine via an organocatalytic Diels–Alder approach. Nat. Commun. 6:7204 doi: 10.1038/ncomms8204 (2014).
References
Sonnenschein, F. L. Ueber einige bestandtheile von gelsemium sempervirens. Ber. Dtsch. Chem. Ges. 9, 1182–1186 (1876).
Conroy, H. & Chakrabarti, J. K. NMR spectra of gelsemine derivatives. The structure and biogenesis of the alkaloid gelsemine. Tetrahedron Lett. 1, 6–13 (1959).
Schun, Y. & Cordell, G. A. Studies on the NMR spectroscopic properties of gelsemine - revisions and refinements. J. Nat. Prod. 48, 969–971 (1985).
Lovell, F. M., Pepinsky, R. & Wilson, A. J. C. X-ray analysis of the structure of gelsemine hydrohalides. Tetrahedron Lett. 1, 1–5 (1959).
Schun, Y., Cordell, G. A. & Garland, M. 21-oxogelsevirine, a new alkaloid from Gelsemium rankinii. J. Nat. Prod. 49, 483–487 (1986).
Schun, Y. & Cordell, A. G. Rankinidine, a new indole alkaloid from Gelsemium rankinii. J. Nat. Prod. 49, 806–808 (1986).
Ponglux, D. et al. Studies on the indole alkaloids of gelsemium elegans (Thailand): structure elucidation and proposal of biogenetic route. Tetrahedron 44, 5075–5094 (1988).
Lin, L. Z., Schun, Y., Cordell, A. G., Ni, C.-Z. & Clardy, J. Three oxindole alkaloids from Gelsemium species. Phytochemistry 30, 679–683 (1991).
Autrey, R. L. & Tahk, F. C. The synthesis and stereochemistry of some isatylideneacetic acid derivatives. Tetrahedron 23, 901–917 (1967).
Autrey, R. L. & Tahk, F. C. Oxindoles-II: the products of some Michael additions to isatylideneacetic esters and cinnamyl derivatives. Tetrahedron 24, 3337–3345 (1968).
Johnson, R. S., Lovett, T. O. & Stevens, T. S. The alkaloids of Gelsemium sempervirens. Part IV. Derivatives of pyridine, isoquinoline, and indol-2(3H)-one as possible initial materials for synthesis of gelsemine. J. Chem. Soc. C, 796–800 (1970).
Fleming, I., Loreto, M. A., Michael, J. P. & Wallace, I. H. M. Two new stereochemically complementary oxindole synthesis. Tetrahedron Lett. 23, 2053–2056 (1982).
Fleming, I., Loreto, M. A., Wallace, I. H. M. & Michael, J. P. Two new oxindole syntheses. J. Chem. Soc. Perkin Trans. 1 349–359 (1986).
Stork, G., Krafft, M. E. & Biller, S. A. An approach to gelsemine. Tetrahedron Lett. 28, 1035–1038 (1987).
Vijn, R. J., Hiemstra, H., Kok, J. J., Knotter, M. & Speckamp, W. N. Synthetic studies towards gelsemine, I. The importance of the antiperiplanar effect in the highly regioselective reduction of non-symmetrical cis-hexahydrophthalimides. Tetrahedron 43, 5019–5030 (1987).
Abelman, M. M., Oh, T. & Overman, L. E. Intramolecular alkene arylations for rapid assembly of polycyclic systems containing quaternary centers. A new synthesis of spirooxindoles and other fused and bridged ring systems. J. Org. Chem. 52, 4130–4133 (1987).
Clarke, C. et al. An approach to the synthesis of gelsemine: the intramolecular reaction of an allylsilane with an acyliminium ion for the synthesis of one of the quaternary centres. Tetrahedron 44, 3931–3934 (1988).
Hiemstra, H., Vijn, R. J. & Speckamp, W. N. Synthetic studies toward gelsemine. 2. Preparation of the tetracyclic skeletal part by way of a highly stereospecific intramolecular reaction of a silyl enol ether with an N-acyliminium ion. J. Org. Chem. 53, 3882–3884 (1988).
Earley, W. G., Jacobsen, E. J., Meier, G. P., Oh, T. & Overman, L. E. Synthesis studies directed toward gelsemine. A new synthesis of highly functionalized cis-hydroisoquinolines. Tetrahedron Lett. 29, 3781–3784 (1988).
Earley, W. G., Oh, T. & Overman, L. E. Synthesis studies directed toward gelsemine. Preparation of an advanced pentacyclic intermediate. Tetrahedron Lett. 29, 3785–3788 (1988).
Choi, J. K. et al. α-acylamino radical cyclizations: application to the synthesis of a tetracyclic substructure of gelsemine. J. Org. Chem. 54, 279–290 (1989).
Fleming, I., Moses, R. C., Tercel, M. & Ziv, J. A new oxindole synthesis. J. Chem. Soc. Perkin Trans. 1 617–626 (1991).
Hart, D. J. & Wu, S. C. Intramolecular addition of aryl radicals to vinylogous urethanes: studies toward preparation of the oxindole portion of gelsemine. Tetrahedron Lett. 32, 4099–4102 (1991).
Koot, W.-J., Hiemstra, H. & Speckamp, W. N. (R)-1-acetyl-5-isopropoxy-3-pyrrolin-2-one: a versatile chiral dienophile from (S)-malic acid. J. Org. Chem. 57, 1059–1061 (1992).
Madin, A. & Overman, L. E. Controlling stereoselection in intramolecular heck reactions by tailoring the palladium catalyst. Tetrahedron Lett. 33, 4859–4862 (1992).
Overman, L. E. & Sharp, M. J. Reaction of Na2Fe(CO)4 with an unsaturated aziridinium ion. Unprecedented rearrangement of an alkyltetracarbonylferrate intermediate. J. Org. Chem. 57, 1035–1038 (1992).
Hart, D. J. & Wu, S. C. Gelsemine model studies: alkoxymethylations of decalones and indoles. Heterocycles 35, 135–138 (1993).
Takayama, H., Seki, N., Kitajima, M., Aimi, N. & Sakai, S.-I. Application of Tungstate-catalyzed oxidation to the conversion of oxindoles into the corresponding Na-methoxyoxindoles in the gelsemium alkaloid synthesis. Nat. Prod. Lett. 2, 271–276 (1993).
Johnson, A. P., Luke, R. W. A., Steele, R. W. & Boa, A. N. Synthesis of 1-acylamino-1-(trimethylsiloxy)alkanes by trimethylsilyl trifluoromethanesulfonate-catalysed addition of bis(trimethylsilyl)amides to aldehydes. J. Chem. Soc. Perkin Trans. 1 883–893 (1996).
Ng, F., Chiu, P. & Danishefsky, S. J. Toward a potential total synthesis of gelsemine: a regioselective hydroboration directed by a remote olefin. Tetrahedron Lett. 39, 767–770 (1998).
Sung, M. J., Lee, C.-W. & Cha, J. K. Ti(II)-mediated cyclization of ω-vinylimides. A stereoselective approach to gelsemine. Synlett 561–562 (1999).
Avent, A. G., Byrne, P. W. & Pankett, C. S. A photochemical approach to the gelsemine skeleton. Org. Lett. 1, 2073–2075 (1999).
Dijkink, J., Cintrat, J.-C., Speckamp, W. N. & Hiemstra, H. Enantioselective synthesis of a key tricyclic intermediate en route to (+)-gelsemine. Tetrahedron Lett. 40, 5919–5922 (1999).
Pearson, A. J. & Wang, X. A convenient one-pot procedure to afford bicyclic molecules by stereospecific iron carbonyl mediated [6+2] ene-type cyclization: a possible approach to gelsemine. J. Am. Chem. Soc. 125, 13326–13327 (2003).
Grecian, S. & Aube, J. Double conjugate addition of a nitropropionate ester to a quinone monoketal: Synthesis of an advanced intermediate to (±)-gelsemine. Org. Lett. 9, 3153–3156 (2007).
Tchabanenko, K., Simpkins, N. S. & Male, L. A concise approach to a gelsemine core structure using an oxygen to carbon bridge swapping strategy. Org. Lett. 10, 4747–4750 (2008).
Liu, C.-T. & Yu, Q.-S. Biomimetic synthesis of koumine. Acta Chim. Sinica 45, 359–364 (1987).
Sheikh, Z., Steel, R., Tasker, A. S. & Johnson, A. P. A total synthesis of gelsemine: synthesis of a key tetracyclic intermediate. J. Chem. Soc. Chem. Commun. 763–764 (1994).
Dutton, J. K., Steel, R. W., Tasker, A. S., Popsavin, V. & Johnson, A. P. A total synthesis of gelsemine: oxindole spiroannelation. J. Chem. Soc. Chem. Commun. 765–766 (1994).
Newcombe, N. J., Ya, F., Vijn, R. J., Hiemstra, H. & Speckamp, W. N. The total synthesis of (±)-gelsemine. J. Chem. Soc. Chem. Commun. 767–768 (1994).
Atarashi, S. et al. Free radical cyclizations in alkaloid total synthesis: (±)-21-Oxogelsemine and (±)-gelsemine. J. Am. Chem. Soc. 119, 6226–6241 (1997).
Fukuyama, T. & Liu, G. Stereocontrolled total synthesis of (±)-gelsemine. J. Am. Chem. Soc. 118, 7426–7427 (1996).
Madin, A. et al. Total synthesis of (±)-gelsemine. Angew. Chem. Int. Ed. 38, 2934–2936 (1999).
Yokoshima, S., Tokuyama, H. & Fukuyama, T. Enantioselective total synthesis of (+)-gelsemine: determination of its absolute configuration. Angew. Chem. Int. Ed. 39, 4073–4075 (2000).
Ng, F. W., Lin, H. & Danishefsky, S. J. Explorations in organic chemistry leading to the total synthesis of (±)-gelsemine. J. Am. Chem. Soc. 124, 9812–9824 (2002).
Earley, W. C. et al. Aza-Cope rearrangement-Mannich cyclizations for the formation of complex tricyclic amines: stereocontrolled total synthesis of (±)-gelsemine. J. Am. Chem. Soc. 127, 18046–18053 (2005).
Madin, A. et al. Use of the intramolecular Heck reaction for forming congested quaternary carbon stereocenters: stereocontrolled total synthesis of (±)-gelsemine. J. Am. Chem. Soc. 127, 18054–18065 (2005).
Zhou, X., Xiao, T., Iwama, Y. & Qin, Y. Biomimetic total synthesis of (+)-gelsemine. Angew. Chem. Int. Ed. 51, 4909–4912 (2012).
Lin, H. & Danishefsky, S. J. Gelsemine: a thought-provoking target for total synthesis. Angew. Chem. Int. Ed. 42, 36–51 (2003).
Zhang, J.-Y., Gong, N., Huang, J.-L., Guo, L.-C. & Wang, Y.-X. Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal α3 glycine receptors. Pain 154, 2452–2462 (2013).
Nising, C. F. & Bräse, S. The oxa-Michael reaction: from recent developments to applications in natural product synthesis. Chem. Soc. Rev. 37, 1218–1228 (2008).
Nasir, N. M., Ermanis, K. & Clarke, P. A. Strategies for the construction of tetrahydropyran rings in the synthesis of natural products. Org. Biomol. Chem. 12, 3323–3335 (2014).
Guillena, G., Nájera, C. & Ramón, D. J. Enantioselective direct aldol reaction: the blossoming of modern organocatalysis. Tetrahedron Asymmetry 18, 2249–2293 (2007).
Xiao, Y.-C. & Chen, Y.-C. Intramolecular reactions. Comprehensive Enantioselective Organocatalysis 3, 1069–1090 (2013).
Maria da, C. F. O., Leonardo, S. S. & Ronaldo, A. P. Diastereoselection of the addition of silyloxyfurans to five-, six- and seven-membered N-acyliminium ions. Tetrahedron Lett. 42, 6995–6997 (2001).
Kolonko, J. K. & Reich, H. J. Stabilization of ketone and aldehyde enols by formation of hydrogen bonds to phosphazene enolates and their aldol products. J. Am. Chem. Soc. 130, 9668–9669 (2008).
Denmark, S. E. & Henke, B. R. Investigations on transition-state geometry in the aldol condensation. J. Am. Chem. Soc. 113, 2177–2194 (1991).
Nakano, H. et al. A novel chiral oxazolidine organocatalyst for the synthesis of an oseltamivir intermediate using a highly enantioselective Diels-Alder reaction of 1,2-dihydropyridine. Chem. Commun. 46, 4827–4829 (2010).
Kohari, Y. et al. Enantioselective Diels-Alder reaction of 1,2-dihydropyridines with aldehydes using β-amino alcohol organocatalyst. J. Org. Chem. 79, 9500–9511 (2014).
Bayly, A. R., White, A. J. P. & Spivey, A. C. Design and synthesis of a prototype scaffold for five-residue α-helix mimetics. Eur. J. Org. Chem. 25, 5566–5569 (2013).
Kraus, G. A. & Kim, J. Tandem Diels-Alder/ene reactions. Org. Lett. 6, 3115–3117 (2004).
Sato, T. & Otera, J. CsF in organic synthesis. Malonic ester synthesis revisited for stereoselective carbon-carbon bond formation. J. Org. Chem. 60, 2627–2629 (1995).
Acknowledgements
H.Z. thanks the National Basic Research Program of China (973 Program: 2010CB833200), NSFC (21172100; 21272105; 21290183), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: IRT1138), FRFCU (lzujbky-2013-ct02) and ‘111’ Program of MOE for financial support.
Author information
Authors and Affiliations
Contributions
F.G.Q. and H.Z. conceived the synthetic design and directed the project. X.C., S.D. and C.T. conducted the experimental work and data analysis. F.G.Q. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-16, Supplementary Methods and Supplementary References (PDF 7435 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, X., Duan, S., Tao, C. et al. Total synthesis of (+)-gelsemine via an organocatalytic Diels–Alder approach. Nat Commun 6, 7204 (2015). https://doi.org/10.1038/ncomms8204
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms8204
This article is cited by
-
Asymmetric construction of all-carbon quaternary stereocenters in the total synthesis of natural products
Science China Chemistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.