Abstract
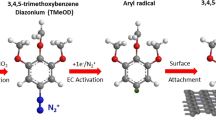
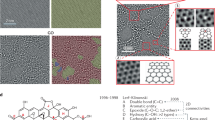
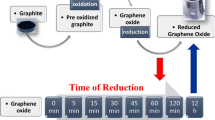
Graphene, a truly two-dimensional and fully π-conjugated honeycomb carbon network, is currently evolving into the most promising successor to silicon in micro- and nanoelectronic applications. However, its wider application is impeded by the difficulties in opening a bandgap in its gapless band-structure, as well as the lack of processability in the resultant intrinscially insoluble material. Covalent chemical modification of the π-electron system is capable of addressing both of these issues through the introduction of variable chemical decoration. Although there has been significant research activity in the field of functionalized graphene, most work to date has focused on the use of graphene oxide. In this Article, we report on the first wet chemical bulk functionalization route beginning with pristine graphite that does not require initial oxidative damage of the graphene basal planes. Through effective reductive activation, covalent functionalization of the charged graphene is achieved by organic diazonium salts. Functionalization was observed spectroscopically, and successfully prevents reaggregation while providing solubility in common organic media.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Novoselov, K. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Allen, M., Tung, V. & Kaner, R. Honeycomb carbon: a review of graphene. Chem. Rev. 110, 132–145 (2010).
Park, S. & Ruoff, R. S. Chemical methods for the production of graphenes. Nature Nanotech. 4, 217–224 (2009).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nature Nanotech. 5, 574–578 (2010).
Emtsev, K. V. et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nature Mater. 8, 203 (2009).
Englert, J. M. et al. Soluble graphene: generation of aqueous graphene solutions aided by a perylenebisimide-based bolaamphiphile. Adv. Mater. 21, 4265–4269 (2009).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotech. 3, 563–568 (2008).
Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Deutsch. Chem. Ges. 32, 1394–1399 (1899).
Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958).
Lotya, M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009).
Liu, H. et al. Photochemical reactivity of graphene. J. Am. Chem. Soc. 131, 17099–17101 (2009).
Bekyarova, E. et al. Chemical modification of epitaxial graphene: spontaneous grafting of aryl groups. J. Am. Chem. Soc. 131, 1336–1337 (2009).
Lomeda, J. R., Doyle, C. D., Kosynkin, D. V., Hwang, W-F. & Tour, J. M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 130, 16201–16206 (2008).
Pan, Q., Wang, H. & Jiang, Y. Covalent modification of natural graphite with lithium benzoate multilayers via diazonium chemistry and their application in lithium ion batteries. Electrochem. Commun. 9, 754–760 (2007).
Sharma, R., Baik, J. H., Perera, C. J. & Strano, M. S. Anomalously large reactivity of single graphene layers and edges toward electron transfer chemistries. Nano Lett. 10, 398 (2010).
Sun, Z., Kohama, S-i., Zhang, Z., Lomeda, J. R. & Tour, J. M. Soluble graphene through edge-selective functionalization. Nano Res. 3, 117 (2010).
Zhu, Y., Higginbotham, A. L. & Tour, J. M. Covalent functionalization of surfactant-wrapped graphene nanoribbons. Chem. Mater. 21, 5284–5291 (2009).
Kudin, K. N. et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8, 36–41 (2008).
Syrgiannis, Z. et al. Reductive retrofunctionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 49, 3322–3325 (2010).
Liang, F. et al. A convenient route to functionalized carbon nanotubes. Nano Lett. 4, 1257–1260 (2004).
Billups, W. E., Liang, F., Chattopadhyay, J. & Beach, J. M. Uses of single wall carbon nanotube salts in organic syntheses. ECS Trans. 2, 65–76 (2007).
Wunderlich, D., Hauke, F. & Hirsch, A. Preferred functionalization of metallic and small-diameter single walled carbon nanotubes via reductive alkylation. J. Mater. Chem. 18, 1493–1497 (2008).
Haddon, R. C. π-electrons in three dimensions. Acc. Chem. Res. 21, 243–249 (1988).
Haddon, R. C. C60: sphere or polyhedron? J. Am. Chem. Soc. 119, 1797–1798 (1997).
Chen, Z., Thiel, W. & Hirsch, A. Reactivity of the convex and concave surfaces of single-walled carbon nanotubes (SWCNTs) towards addition reactions: dependence on the carbon-atom pyramidalization. ChemPhysChem 4, 93–97 (2003).
Stephenson, J. J., Sadana, A. K., Higginbotham, A. L. & Tour, J. M. Highly functionalized and soluble multiwalled carbon nanotubes by reductive alkylation and arylation: the Billups reaction. Chem. Mater. 18, 4658–4661 (2006).
Strano, M. S. et al. Electronic structure control of single-walled carbon nanotube functionalization. Science 301, 1519–1522 (2003).
Sinitskii, A. et al. Kinetics of diazonium functionalization of chemically converted graphene nanoribbons. ACS Nano 4, 1949–1954 (2010).
Sharma, R., Nair, N. & Strano, M. S. Structure–reactivity relationships for graphene nanoribbons. J. Phys. Chem. C 113, 14771–14777 (2009).
Usrey, M. L., Lippmann, E. S. & Strano, M. S. Evidence for a two-step mechanism in electronically selective single-walled carbon nanotube reactions. J. Am. Chem. Soc. 127, 16129–16135 (2005).
Fantini, C., Pimenta, M. A. & Strano, M. S. Two-phonon combination Raman modes in covalently functionalized single-wall carbon nanotubes. J. Phys. Chem. C 112, 13150–13155 (2008).
Rüdorff, W. Einlagerungsverbindungen mit Alkali- und Erdalkalimetallen. Angew. Chem. 71, 487–491 (1959).
Rüdorff, W. & Schulze, E. Über Alkaligraphitverbindungen. Z. Anorg. Allg. Chem. 277, 156–171 (1954).
Ginderow, D. Preparation of insertion compounds in graphite from liquid reagents. Ann. Chim. 6, 5–16 (1971).
Vallés, C. et al. Solutions of negatively charged graphene sheets and ribbons. J. Am. Chem. Soc. 130, 15802–15804 (2008).
Allongue, P. et al. Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 119, 201–207 (1997).
Viculis, L. M., Mack, J. J. & Kaner, R. B. A chemical route to carbon nanoscrolls. Science 299, 1361 (2003).
Schniepp, H. C. et al. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 110, 8535–8539 (2006).
Erickson, K. et al. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 22, 4467–4472 (2010).
Ferrari, A. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 (2006).
Abergel, D. S. L., Russell, A. & Fal'ko, V. I. Visibility of graphene flakes on a dielectric substrate. Appl. Phys. Lett. 91, 063125 (2007).
Blake, P. et al. Making graphene visible. Appl. Phys. Lett. 91, 063124 (2007).
Solin, S. A. Raman and IR studies of intercalated graphite. Physica 99B, 443–453 (1980).
Berciaud, S., Ryu, S., Brus, L. E. & Heinz, T. F. Probing the intrinsic properties of exfoliated graphene: Raman spectroscopy of free-standing monolayers. Nano Lett. 9, 346–352 (2009).
Nemanich, R. J., Glass, J. T., Lucovsky, G. & Shroder, R. E. Raman scattering characterization of carbon bonding in diamond and diamondlike thin films. J. Vac. Sci. Technol. A 6, 1783–1787 (1988).
Ferrari, A. C. & Robertson, J. Origin of the 1150 cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 63, 121405 (2001).
Graf, D. et al. Spatially resolved Raman spectroscopy of single- and few-layer graphene. Nano Lett. 7, 238–242 (2007).
Basko, D. M. Effect of inelastic collisions on multiphonon Raman scattering in graphene. Phys. Rev. B 76, 081405 (2007).
Jung, N. et al. Charge transfer chemical doping of few layer graphenes: charge distribution and band gap formation. Nano Lett. 9, 4133–4137 (2009).
Lindberg, B. J. et al. Molecular spectroscopy by means of ESCA. II. Sulfur compounds. Correlation of electron binding energy with structure. Phys. Scripta 1, 286 (1970).
Ruangchuay, L., Schwank, J. & Sirivat, A. Surface degradation of α-naphthalene sulfonate-doped polypyrrole during XPS characterization. Appl. Surf. Sci. 199, 128 (2002).
Tour, J. M. et al. Self-assembled monolayers and multilayers of conjugated thiols, α,w-dithiols, and thioacetyl-containing adsorbates. Understanding attachments between potential molecular wires and gold surfaces. J. Am. Chem. Soc. 117, 9529 (1995).
Buchner, F. et al. Coordination of iron atoms by tetraphenylporphyrin monolayers and multilayers on Ag(111) and formation of iron-tetraphenylporphyrin. J. Phys. Chem. C 112, 15458 (2008).
Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG), the Interdisciplinary Center for Molecular Materials (ICMM), the European Research Council (ERC; grant 246622 — GRAPHENOCHEM), the Graduate School Molecular Science (GSMS) and the Cluster of Excellence ‘Engineering of Advanced Materials (EAM)’ for financial support.
Author information
Authors and Affiliations
Contributions
F.H. and A.H. supervised the project as scientific group leader and principal investigator. J.M.E. worked out the concept, synthesized all compounds, carried out Raman, absorption, emission, IR and NMR spectroscopy, and performed AFM, SRM and optical microscopy. C.D. carried out TGA/MS characterization. E.S. and G.Y. conducted HRTEM and EDX investigations. M.S., C.P., J.M.G. and H.-P.S. carried out XPS analysis. A.H., F.H. and J.M.E. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4207 kb)
Rights and permissions
About this article
Cite this article
Englert, J., Dotzer, C., Yang, G. et al. Covalent bulk functionalization of graphene. Nature Chem 3, 279–286 (2011). https://doi.org/10.1038/nchem.1010
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1010
This article is cited by
-
Chemomechanical modification of quantum emission in monolayer WSe2
Nature Communications (2023)
-
Atomically resolved TEM imaging of covalently functionalised graphene
npj 2D Materials and Applications (2022)
-
Azobenzene-Based Solar Thermal Fuels: A Review
Nano-Micro Letters (2022)
-
Silane-functionalized graphene nanoplatelets for silicone rubber nanocomposites
Journal of Materials Science (2022)