Abstract
TNF is a master proinflammatory cytokine whose pathogenic role in inflammatory disorders can, in certain conditions, be attributed to RIPK1 kinase-dependent cell death. Survival, however, is the default response of most cells to TNF stimulation, indicating that cell demise is normally actively repressed and that specific checkpoints must be turned off for cell death to proceed. We identified RIPK1 as a direct substrate of MK2 in the TNFR1 signalling pathway. Phosphorylation of RIPK1 by MK2 limits cytosolic activation of RIPK1 and the subsequent assembly of the death complex that drives RIPK1 kinase-dependent apoptosis and necroptosis. In line with these in vitro findings, MK2 inactivation greatly sensitizes mice to the cytotoxic effects of TNF in an acute model of sterile shock caused by RIPK1-dependent cell death. In conclusion, we identified MK2-mediated RIPK1 phosphorylation as an important molecular mechanism limiting the sensitivity of the cells to the cytotoxic effects of TNF.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Croft, M., Benedict, C. A. & Ware, C. F. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 12, 147–168 (2013).
Berger, S. B. et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014).
Rickard, J. A. et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife 3, e03464 (2014).
Vlantis, K. et al. NEMO prevents RIP kinase 1-mediated epithelial cell death and chronic intestinal inflammation by NF-κB-dependent and -independent functions. Immunity 44, 553–567 (2016).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Ting, A. T. & Bertrand, M. J. More to life than NF-κB in TNFR1 signaling. Trends Immunol. 37, 535–545 (2016).
Van Antwerp, D. J., Martin, S. J., Kafri, T., Green, D. R. & Verma, I. M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274, 787–789 (1996).
Liu, Z. G., Hsu, H., Goeddel, D. V. & Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87, 565–576 (1996).
Peltzer, N., Darding, M. & Walczak, H. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 26, 445–461 (2016).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Kanayama, A. et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 (2004).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Draber, P. et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 13, 2258–2272 (2015).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Wilson, N. S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat. Immunol. 10, 348–355 (2009).
Dondelinger, Y. et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell 60, 63–76 (2015).
Dondelinger, Y. et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 20, 1381–1392 (2013).
O’Donnell, M. A., Legarda-Addison, D., Skountzos, P., Yeh, W. C. & Ting, A. T. Ubiquitination of RIP1 regulates an NF-κB-independent cell-death switch in TNF signaling. Curr. Biol. 17, 418–424 (2007).
O’Donnell, M. A., Hase, H., Legarda, D. & Ting, A. T. NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1. PLoS ONE 7, e41238 (2012).
Petersen, S. L. et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 (2007).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Cargnello, M. & Roux, P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 (2011).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Zhang, Y. et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 8, 14329 (2017).
de Almagro, M. C. et al. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ. 24, 26–37 (2016).
Boutaffala, L. et al. NIK promotes tissue destruction independently of the alternative NF-κB pathway through TNFR1/RIP1-induced apoptosis. Cell Death Differ. 22, 2020–2033 (2015).
Vince, J. E. et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFα. J. Cell Biol. 182, 171–184 (2008).
Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011).
Newton, K. et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016).
Takahashi, N. et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 3, e437 (2012).
Degterev, A., Maki, J. L. & Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 20, 366 (2013).
Legarda-Addison, D., Hase, H., O’Donnell, M. A. & Ting, A. T. NEMO/IKKγ regulates an early NF-κB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 16, 1279–1288 (2009).
Koppe, C. et al. IκB kinaseα/β control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1. Hepatology 64, 1217–1231 (2016).
Lalaoui, N. et al. Targeting p38 or MK2 enhances the anti-leukemic activity of Smac-mimetics. Cancer Cell 30, 499–500 (2016).
Ben-Levy, R., Hooper, S., Wilson, R., Paterson, H. F. & Marshall, C. J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 8, 1049–1057 (1998).
Engel, K., Kotlyarov, A. & Gaestel, M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17, 3363–3371 (1998).
Vandendriessche, B. et al. MAPK-activated protein kinase 2-deficiency causes hyperacute tumor necrosis factor-induced inflammatory shock. BMC Physiol. 14, 5 (2014).
Jaco, I. et al. MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol. Cell 66, 698–710 (2017).
Silke, J., Rickard, J. A. & Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 16, 689–697 (2015).
Menon, M. et al. p38MAPK/MK2-dependent phosphorylation controls cytotoxic RIPK1 signaling in inflammation and infection. Nat. Cell Biol. https://doi.org/10.1038/ncb3614 (2017).
Dejardin, E. et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17, 525–535 (2002).
Ronkina, N. et al. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 27, 170–181 (2007).
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 (2005).
Acknowledgements
Research in the groups of M.J.M.B. and P.V. is supported by grants from the Vlaams Instituut voor Biotechnologie (VIB), from the ‘Foundation against Cancer’ (2012-188 and FAF-F/2016/865), from Ghent University (MRP, GROUP-ID consortium), from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO) (G017212N, G013715N, G078713N), from the Flemish Government (Methusalem BOF09/01M00709 and BOF16/MET_V/007), and from the Belgian science policy office (BELSPO) (IAP 7/32). Y.D. was paid by the agentschap voor Innovatie door Wetenschap en Technologie (IWT), followed by FWO grant G017212N and G013715N, and Methusalem. T.D. and D.P. have a strategic basic research PhD fellowship from the FWO. D.R.-R. is paid by FWO grant G013715N and T.D. is financed by the FWO grant G0A5413N and G0C3714N and Methusalem. F.V.H. was paid by European Consortium Apo-Sys (FP7-HEALTH-2007-A-200767). We thank M. Gaestel (Hannover Medical School, Germany) for the MK2-deficient MEFs and W. Declercq (VIB-UGent) for constructive discussions.
Author information
Authors and Affiliations
Contributions
Y.D. and M.J.M.B. designed the study. Y.D., T.D., D.R.-R., D.P. and M.J.M.B. designed the experiments. Y.D., T.D., D.R.-R., D.P., T.D., I.B., F.V.H. and M.J.M.B. performed the experiments. M.J.M.B. wrote the manuscript. Y.D., T.D. and P.V. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
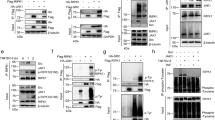
Supplementary Figure 1 TNF-induced phosphorylation of RIPK1 in the cytosol is 2 MK2-dependent.
WT MEFs (a–c) and MEFs of the indicated genotypes (d) where 3 preincubated with the indicated compounds for 30 min before stimulation with 20 ng/ml 4 hTNF for the indicated amount of time. Protein levels and RIPK1 phosphorylation were 5 determined by immunoblotting. (e) Proposed model of MK2 activation and MK2-dependent 6 RIPK1 phosphorylation. Unprocessed original scans of blots are shown in Supplementary 7 Fig. 7. The representative results shown were confirmed in at least two independent 8 experiments. 9 10
Supplementary Figure 2 MK2-mediated phosphorylation of RIPK1 does not affect 11 TNF-induced signaling to NF-κB or MAPKs.
(a) WT MEFs were preincubated with MK2i 12 when indicated for 30 min and subsequently stimulated with 2 μg/ml FLAG-hTNF. TNFR1 13 complex I was then FLAG immunoprecipitated. (b–f) WT MEFs were treated with 20 ng/ml 14 hTNF in the presence or absence of the MK2 inhibitor for the indicated time. (c–f) RNA was 15 isolated and relative mRNA levels were determined by RT-PCR. RT-PCR results are presented as the mean of n = 2 or 3 independent experiments. (g) Ripk1−/− 16 MEFs lentivirally 17 reconstituted with full length RIPK1 (WT) or the quadruple Ser321Ala Ser332Ala Ser334Ala 18 Ser336Ala RIPK1 mutant (4SA) were stimulated with 20 ng/ml hTNF for the indicated time. 19 Proteins levels were determined by immunoblotting (a,b,g). Unprocessed original scans of 20 blots are shown in Supplementary Fig. 7. Raw data from independent experiments are 21 provided in Supplementary Table 1. The representative results shown were confirmed in at 22 least two independent experiments. 23 24
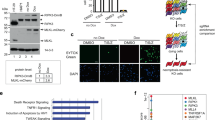
Supplementary Figure 3 MK2 inhibition in MEFs and BMDMs results in increased Caspase-3 activity following TNF stimulation in IKK inactivated conditions.
(a) Ikkα−/− 25 Ikkβ−/− 26 MEFs were pretreated for 30 min with the indicated compounds and then treated with 27 80 pg/ml hTNF. (b) WT BMDMs were pretreated for 30 min with the indicated compounds 28 and then treated with 100 pg/ml hTNF. Caspase activity was measured in function of time by 29 DEVD-AMC fluorescence. The results presented in (a) are representative of n = 3 independent 30 experiments. The results presented in (b) represent the mean ± s.e.m. of n = 3 independent 31 experiments. Statistical significance was determined using two-way ANOVA followed by a 32 Tukey post-hoc test. Significance between samples is indicated in the figures as followed: ∗: 33 p < 0.05; ∗∗: p < 0.01; ∗∗∗: p < 0.001; NS, non-significant. Raw data from independent 34 experiments are provided in Supplementary Table 1. 35 36
Supplementary Figure 4 MK2 inhibition facilitates recruitment of RIPK1 to complex IIb/necrosome.
(a–d) Ikkα−/− Ikkβ−/− 37 MEFs were pretreated for 30 min with indicated 38 compounds and stimulated with 20 ng/ml hTNF for indicated time points (a,c) or for 1 hour 39 (b) or 2 h (c). Complex IIb/necrosome was isolated by FADD immunoprecipitation 40 followed by USP2 treatment and/or lambda phosphatase (PPase) when indicated. Protein 41 levels were determined by immunoblotting. Ubiquitylation of RIPK1 (b) or MK2 42 phosphorylation of RIPK1 (c) is shown. Unprocessed original scans of blots are shown in 43 Supplementary Fig. 7. The representative results shown were confirmed in at least two 44 independent experiments. 45 46
Supplementary Figure 5 MK2 protects mice from TNF-induced RIPK1 kinase- 47 dependent lethal hypothermia.
C57BL/6J female mice were injected with the indicated 48 compounds 15 min prior to a challenge with 5 μg mTNF per 20 g of body weight. (a–c) Body 49 temperature were determined in function of time. Dead mice are considered to be at 22 °C. 50 These graphs are dot plot representations of the results presented in Fig. 6g. Raw data from 51 independent experiments are provided in Supplementary Table 1. Vehicle (n = 9 mice), Nec-1s 52 (n = 5 mice), MK2i (n = 9 mice) and MK2i + Nec-1s (n = 9 mice). 53 54
Supplementary Figure 6 Increased cell death resulting from MK2 inhibition is not 55 originating from autocrine production of TNF.
WT MEFs (a,b), GFP- or MK2- reconstituted Mapkapk2−/− MEFs (c), Ripk1−/− 56 MEFs lentivirally reconstituted with full length 57 RIPK1 (WT) or the quadruple Ser321Ala Ser332Ala Ser334Ala Ser336Ala RIPK1 mutant 58 (4SA) (d) were pretreated with the murine specific anti-TNF blocking antibody XT-22 for 30 59 min when indicated and stimulated with either 750 pg/ml (a,b), 0.33 pg/ml (c) or 80 pg/ml 60 (d) hTNF. Cell death was measured in function of time by SytoxGreen positivity. Cell death 61 data are presented as mean ± s.e.m. of n independent experiments (n = 3 independent 62 experiments for a,d) (n = 4 independent experiments for b) (n = 5 independent experiments for 63 c). Statistical significance was determined using two-way ANOVA followed by a Tukey 64 post-hoc test. Significance between samples is indicated in the figures as followed: ∗ : p < 65 0.05; ∗∗: p < 0.01; ∗∗∗: p < 0.001; NS, non-significant. Raw data from independent 66 experiments are provided in Supplementary Table 1. 67 68
Supplementary information
Supplementary Information
Supplementary Information (PDF 65330 kb)
Supplementary Table 1
Supplementary Information (XLSX 86 kb)
Rights and permissions
About this article
Cite this article
Dondelinger, Y., Delanghe, T., Rojas-Rivera, D. et al. MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat Cell Biol 19, 1237–1247 (2017). https://doi.org/10.1038/ncb3608
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3608