Abstract
Remodelling of the human embryo at implantation is indispensable for successful pregnancy. Yet it has remained mysterious because of the experimental hurdles that beset the study of this developmental phase. Here, we establish an in vitro system to culture human embryos through implantation stages in the absence of maternal tissues and reveal the key events of early human morphogenesis. These include segregation of the pluripotent embryonic and extra-embryonic lineages, and morphogenetic rearrangements leading to generation of a bilaminar disc, formation of a pro-amniotic cavity within the embryonic lineage, appearance of the prospective yolk sac, and trophoblast differentiation. Using human embryos and human pluripotent stem cells, we show that the reorganization of the embryonic lineage is mediated by cellular polarization leading to cavity formation. Together, our results indicate that the critical remodelling events at this stage of human development are embryo-autonomous, highlighting the remarkable and unanticipated self-organizing properties of human embryos.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
17 May 2016
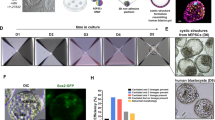
In the version of this Technical Report originally published online, in Fig. 1d (which presents pilot in vitro culture experiments) it erroneously stated that 20% HCS was used in the IVC2 medium; it should have stated that 30% KSR was used. This error arose by a miscommunication between the postdoctoral fellow who contributed to the pilot in vitro culture experiments and prepared the media, and the postdoctoral fellow who performed the experiments. The composition of the media has been verified based on the laboratory notebooks that describe the media preparation. This error has been corrected in the labels and caption of Fig. 1d, in the description of the results in the main text, and in the Methods section, in all versions of the Technical Report.
References
Edwards, R. G., Bavister, B. D. & Steptoe, P. C. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature 221, 632–635 (1969).
Edwards, R. G., Steptoe, P. C. & Purdy, J. M. Fertilization and cleavage in vitro of preovulator human oocytes. Nature 227, 1307–1309 (1970).
Koot, Y. E., Teklenburg, G., Salker, M. S., Brosens, J. J. & Macklon, N. S. Molecular aspects of implantation failure. Biochim. Biophys. Acta 1822, 1943–1950 (2012).
Enders, A. C., Schlafke, S. & Hendrickx, A. G. Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am. J. Anat. 177, 161–185 (1986).
Pera, M. F. & Trounson, A. O. Human embryonic stem cells: prospects for development. Development 131, 5515–5525 (2004).
Weimar, C. H., Post Uiterweer, E. D., Teklenburg, G., Heijnen, C. J. & Macklon, N. S. In-vitro model systems for the study of human embryo-endometrium interactions. Reprod. Biomed. Online 27, 461–476 (2013).
Bedzhov, I., Leung, C. Y., Bialecka, M. & Zernicka-Goetz, M. In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 9, 2732–2739 (2014).
Bedzhov, I. & Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032–1044 (2014).
Hertig, A. T., Rock, J. & Adams, E. C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 98, 435–493 (1956).
Hur, Y. S. et al. Effect of artificial shrinkage on clinical outcome in fresh blastocyst transfer cycles. Clin. Exp. Reprod. Med. 38, 87–92 (2011).
Morris, S. A. et al. Dynamics of anterior-posterior axis formation in the developing mouse embryo. Nat. Commun. 3, 673 (2012).
Pera, M. F. et al. What if stem cells turn into embryos in a dish? Nat. Methods 12, 917–919 (2015).
Gardner, D. K. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod. Biomed. Online 32, 137–141 (2015).
Covello, K. L. et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20, 557–570 (2006).
Ezashi, T., Das, P. & Roberts, R. M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl Acad. Sci. USA 102, 4783–4788 (2005).
Rivera-Perez, J. A., Jones, V. & Tam, P. P. Culture of whole mouse embryos at early postimplantation to organogenesis stages: developmental staging and methods. Methods Enzymol. 476, 185–203 (2010).
Bedzhov, I., Graham, S. J., Leung, C. Y. & Zernicka-Goetz, M. Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Phil. Trans. R. Soc. B 369 (2014).
Roode, M. et al. Human hypoblast formation is not dependent on FGF signalling. Dev. Biol. 361, 358–363 (2012).
Teklenburg, G. et al. Cell lineage specific distribution of H3K27 trimethylation accumulation in an in vitro model for human implantation. PLoS ONE 7, e32701 (2012).
Niakan, K. K. & Eggan, K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 375, 54–64 (2013).
O’Leary, T. et al. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 30, 278–282 (2012).
Niakan, K. K., Han, J., Pedersen, R. A., Simon, C. & Pera, R. A. Human pre-implantation embryo development. Development 139, 829–841 (2012).
Haigh, T., Chen, C., Jones, C. J. & Aplin, J. D. Studies of mesenchymal cells from 1st trimester human placenta: expression of cytokeratin outside the trophoblast lineage. Placenta 20, 615–625 (1999).
Dobreva, M. P., Pereira, P. N., Deprest, J. & Zwijsen, A. On the origin of amniotic stem cells: of mice and men. Int. J. Dev. Biol. 54, 761–777 (2010).
Hill, J. P. The developmental history of the primates. Phil. Trans. R. Soc. Lond. B 221, 45–178 (1932).
Luckett, W. P. The development of primordial and definitive amniotic cavities in early Rhesus monkey and human embryos. Am. J. Anat. 144, 149–167 (1975).
Palis, J. & Yoder, M. C. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29, 927–936 (2001).
Taniguchi, K. et al. Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Rep. 5, 954–962 (2015).
Bryant, D. M. et al. A molecular switch for the orientation of epithelial cell polarization. Dev. Cell 31, 171–187 (2014).
Bryant, D. M. & Mostov, K. E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 (2008).
Vuoristo, S., Jedrusik, A., Shahbazi, M. N. & Zernicka-Goetz, M. Culture of human embryos through implantation stages in vitro. Protoc. Exch. (2016)10.1038/protex.2016.017.
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Lee, G. Y., Kenny, P. A., Lee, E. H. & Bissell, M. J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4, 359–365 (2007).
Faure, E. et al. A workflow to process 3D + time microscopy images of developing organisms and reconstruct their cell lineage. Nat. Commun. 7, 8674 (2016).
Acknowledgements
We are grateful to the patients donating their embryos, colleagues in the M.Z.-G. laboratory, C. Lee (Gurdon Institute), and embryologists at the CARE, Bourn Hall (K. Elder and P. Snell) and Kings College Guy’s Hospital IVF clinics for help and discussions. We thank P. Braude, D. Glover and C. Ogilvie for insightful discussion and I. Bedzhov for help in a pilot experiment. This work was supported by the Wellcome Trust grant to M.Z.-G. Work in the K.K.N. laboratory was supported by The Francis Crick Institute, which receives its core funding from Cancer Research UK, the Medical Research Council and the Wellcome Trust. M.N.S. was initially supported by a Ramon Areces Spanish Foundation Fellowship, and subsequently by an EMBO Postdoctoral Fellowship. S.V. was supported by a Post Doc Pool Grant from the Finnish Cultural Foundation. G.R. was supported by a Newton Fellowship.
Author information
Authors and Affiliations
Contributions
M.N.S., A.J. and S.V. carried out all experiments and data analyses. G.R. analysed microscopy data and generated 3D reconstructions. A.H. prepared illustrations and contributed experimentally. N.M.E.F. and K.K.N. helped with human embryo cultures and contributed experimentally, and L.G.D. helped with human embryo cultures. A.C., S.F., D.I., Y.K. and K.K.N. oversaw and provided human embryos for these studies. M.Z.-G. conceived the project and supervised the study. M.N.S. and M.Z.-G. wrote the manuscript with help from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 3 3D analysis of embryonic and extra-embryonic lineages in human embryos cultured through implantation stages.
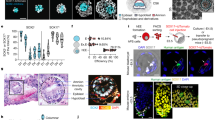
Related to Figs 3 and 4. Human blastocysts developing in vitro until day 11 were fixed and stained at indicated time points. (a) 3D rendering of DAPI stain overlaid with the centre of OCT4-expressing and GATA6-expressing cells. Dots are projected at the back of the volume. (b) Orthogonal views of embryos at different developmental stages stained for aPKC and OCT4. Note the presence of a small pro-amniotic cavity at day 8–9, which is enlarged as development proceeds. All scale bars, 50 μm.
Supplementary Figure 4 Confocal Z-sections of a day 7–8 human embryo.
Related to Fig. 4. Day 7–8 human embryo stained for OCT4, aPKC and F-actin. The different images correspond to representative confocal Z sections. Scale bar, 20 μm. Boxes indicate the magnified area (scale bar, 10 μm).
Supplementary Figure 5 Confocal Z-sections of a day 8–9 human embryo.
Related to Fig. 4. Day 8–9 human embryo stained for OCT4, aPKC and F-actin. The different images correspond to representative confocal Z sections. The arrow indicates the presence of a lumen. Scale bar, 20 μm. Boxes indicate the magnified area (scale bar, 10 μm).
Supplementary Figure 6 Confocal Z-sections of a day 9–10 human embryo.
Related to Fig. 4. Day 9–10 human embryo stained for OCT4, aPKC and F-actin. The different images correspond to representative confocal Z sections. The arrow indicates the presence of a lumen surrounded by radially organised OCT4-expressing epiblast cells. Scale bar, 20 μm. Boxes indicate the magnified area (scale bar, 10 μm).
Supplementary Figure 7 Confocal Z-sections of a day 10–11 human embryo.
Related to Fig. 4. Day 10–11 human embryo stained for OCT4, aPKC and F-actin. The different images correspond to representative confocal Z sections. The arrow indicates the presence of a small lumen surrounded by radially organised OCT4-expressing epiblast cells. Scale bar, 50 μm. Boxes indicate the magnified area (scale bar, 20 μm).
Supplementary information
Supplementary Information
Supplementary Information (PDF 1035 kb)
Development of a day 5 human blastocyst in the in vitro culture system up to day 9–10.
Related to Fig. 1. A day 5 human embryo was cultured in the in vitro culture system for approximately 100 h. Bright field images were taken every 30 min to record its development. Scale bar, 100 μm. (AVI 6989 kb)
Development of a day 9 human blastocyst in the in vitro culture system up to day 12.
Related to Fig. 1. A day 9 human embryo was cultured in the in vitro culture system for approximately 72 h. Bright field images were taken every 30 min to record its development. Scale bar, 100 μm. (AVI 23240 kb)
3D reconstruction of embryonic lineages in a day 9–10 human embryo cultured in vitro.
Related to Fig. 3. Nuclei are shown in blue, OCT4 in grey and GATA6/F-actin in green. (AVI 31620 kb)
3D reconstruction of embryonic lineages in a day 10–11 human embryo cultured in vitro.
Related to Fig. 3. Nuclei are shown in blue, OCT4 in grey and GATA6/F-actin in green. (AVI 32179 kb)
3D reconstruction of the cellular and nuclear shape of representative trophectoderm cells at day 10–11.
Related to Fig. 3. Nuclei are shown in magenta and membranes in green. Note that cells in close proximity to the epiblast have a single nucleus, whereas cells in the periphery of the embryo are multinucleated. (AVI 37380 kb)
3D reconstruction of the pro-amniotic cavity at day 9–10.
Related to Fig. 4. The nuclei of OCT4-expressing epiblast cells is shown in grey and the pro-amniotic cavity in red. (AVI 36620 kb)
3D reconstruction of the cellular shape of representative OCT4-expressing epiblast cells at day 10–11.
Related to Fig. 4. Epiblast cells in close proximity to GATA6-expressing hypoblast cells are shown in green (note the columnar shape characteristic of cells within the epiblast disc). Epiblast cells in close proximity to cytotrophoblast cells are shown in magenta (note the squamous shape characteristic of amniotic cells). (AVI 40836 kb)
3D reconstruction of the prospective yolk sac at day 10–11.
Related to Fig. 4. The nuclei of OCT4-expressing epiblast cells is shown in grey and the prospective yolk sac in blue. (AVI 38440 kb)
3D reconstruction of the hypoblast derived cells and their position with respect to the prospective yolk sac at day 10–11.
Related to Fig. 4. Nuclei of OCT4-expressing epiblast cells are shown in grey, GATA6/F-actin is shown in green and the prospective yolk sac in blue. (AVI 31886 kb)
Rights and permissions
About this article
Cite this article
Shahbazi, M., Jedrusik, A., Vuoristo, S. et al. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 18, 700–708 (2016). https://doi.org/10.1038/ncb3347
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3347